Involvement of Substance P (SP) and Its Related NK1 Receptor in Primary Sjögren's Syndrome (pSS) Pathogenesis
- PMID: 37408182
- PMCID: PMC10216552
- DOI: 10.3390/cells12101347
Involvement of Substance P (SP) and Its Related NK1 Receptor in Primary Sjögren's Syndrome (pSS) Pathogenesis
Abstract
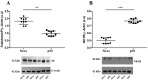
Primary Sjögren's Syndrome (pSS) is a systemic autoimmune disease that primarily attacks the lacrimal and salivary glands, resulting in impaired secretory function characterized by xerostomia and xerophthalmia. Patients with pSS have been shown to have impaired salivary gland innervation and altered circulating levels of neuropeptides thought to be a cause of decreased salivation, including substance P (SP). Using Western blot analysis and immunofluorescence studies, we examined the expression levels of SP and its preferred G protein-coupled TK Receptor 1 (NK1R) and apoptosis markers in biopsies of the minor salivary gland (MSG) from pSS patients compared with patients with idiopathic sicca syndrome. We confirmed a quantitative decrease in the amount of SP in the MSG of pSS patients and demonstrated a significant increase in NK1R levels compared with sicca subjects, indicating the involvement of SP fibers and NK1R in the impaired salivary secretion observed in pSS patients. Moreover, the increase in apoptosis (PARP-1 cleavage) in pSS patients was shown to be related to JNK phosphorylation. Since there is no satisfactory therapy for the treatment of secretory hypofunction in pSS patients, the SP pathway may be a new potential diagnostic tool or therapeutic target.
Keywords: minor salivary gland (MSG); neurokinin receptor 1 (NK1R); primary Sjögren’s syndrome (pSS); sicca syndrome; substance P (SP).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

