Mitochondrial Dysfunction and Impaired Antioxidant Responses in Retinal Pigment Epithelial Cells Derived from a Patient with RCBTB1-Associated Retinopathy
- PMID: 37408192
- PMCID: PMC10216830
- DOI: 10.3390/cells12101358
Mitochondrial Dysfunction and Impaired Antioxidant Responses in Retinal Pigment Epithelial Cells Derived from a Patient with RCBTB1-Associated Retinopathy
Abstract
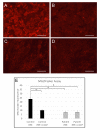
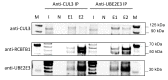
Mutations in the RCBTB1 gene cause inherited retinal disease; however, the pathogenic mechanisms associated with RCBTB1 deficiency remain poorly understood. Here, we investigated the effect of RCBTB1 deficiency on mitochondria and oxidative stress responses in induced pluripotent stem cell (iPSC)-derived retinal pigment epithelial (RPE) cells from control subjects and a patient with RCBTB1-associated retinopathy. Oxidative stress was induced with tert-butyl hydroperoxide (tBHP). RPE cells were characterized by immunostaining, transmission electron microscopy (TEM), CellROX assay, MitoTracker assay, quantitative PCR and immunoprecipitation assay. Patient-derived RPE cells displayed abnormal mitochondrial ultrastructure and reduced MitoTracker fluorescence compared with controls. Patient RPE cells displayed increased levels of reactive oxygen species (ROS) and were more sensitive to tBHP-induced ROS generation than control RPE. Control RPE upregulated RCBTB1 and NFE2L2 expression in response to tBHP treatment; however, this response was highly attenuated in patient RPE. RCBTB1 was co-immunoprecipitated from control RPE protein lysates by antibodies for either UBE2E3 or CUL3. Together, these results demonstrate that RCBTB1 deficiency in patient-derived RPE cells is associated with mitochondrial damage, increased oxidative stress and an attenuated oxidative stress response.
Keywords: RCBTB1; inherited retinal disease; mitochondria; oxidative stress; retinal pigment epithelium.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Coppieters F., Ascari G., Dannhausen K., Nikopoulos K., Peelman F., Karlstetter M., Xu M., Brachet C., Meunier I., Tsilimbaris M.K., et al. Isolated and Syndromic Retinal Dystrophy Caused by Biallelic Mutations in RCBTB1, a Gene Implicated in Ubiquitination. Am. J. Hum. Genet. 2016;99:470–480. doi: 10.1016/j.ajhg.2016.06.017. - DOI - PMC - PubMed
-
- Huang Z., Zhang D., Thompson J.A., Jamuar S.S., Roshandel D., Jennings L., Mellough C., Charng J., Chen S.C., McLaren T.L., et al. Deep clinical phenotyping and gene expression analysis in a patient with RCBTB1-associated retinopathy. Ophthalmic Genet. 2021;42:266–275. doi: 10.1080/13816810.2021.1891551. - DOI - PubMed
-
- Catomeris A.J., Ballios B.G., Sangermano R., Wagner N.E., Comander J.I., Pierce E.A., Place E.M., Bujakowska K.M., Huckfeldt R.M. Novel RCBTB1 variants causing later-onset non-syndromic retinal dystrophy with macular chorioretinal atrophy. Ophthalmic Genet. 2022;43:332–339. doi: 10.1080/13816810.2021.2023196. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

