The RhoA-ROCK1/ROCK2 Pathway Exacerbates Inflammatory Signaling in Immortalized and Primary Microglia
- PMID: 37408199
- PMCID: PMC10216802
- DOI: 10.3390/cells12101367
The RhoA-ROCK1/ROCK2 Pathway Exacerbates Inflammatory Signaling in Immortalized and Primary Microglia
Abstract
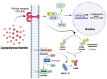
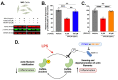
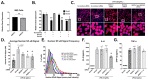
Neuroinflammation is a unifying factor among all acute central nervous system (CNS) injuries and chronic neurodegenerative disorders. Here, we used immortalized microglial (IMG) cells and primary microglia (PMg) to understand the roles of the GTPase Ras homolog gene family member A (RhoA) and its downstream targets Rho-associated coiled-coil-containing protein kinases 1 and 2 (ROCK1 and ROCK2) in neuroinflammation. We used a pan-kinase inhibitor (Y27632) and a ROCK1- and ROCK2-specific inhibitor (RKI1447) to mitigate a lipopolysaccharide (LPS) challenge. In both the IMG cells and PMg, each drug significantly inhibited pro-inflammatory protein production detected in media (TNF-α, IL-6, KC/GRO, and IL-12p70). In the IMG cells, this resulted from the inhibition of NF-κB nuclear translocation and the blocking of neuroinflammatory gene transcription (iNOS, TNF-α, and IL-6). Additionally, we demonstrated the ability of both compounds to block the dephosphorylation and activation of cofilin. In the IMG cells, RhoA activation with Nogo-P4 or narciclasine (Narc) exacerbated the inflammatory response to the LPS challenge. We utilized a siRNA approach to differentiate ROCK1 and ROCK2 activity during the LPS challenges and showed that the blockade of both proteins may mediate the anti-inflammatory effects of Y27632 and RKI1447. Using previously published data, we show that genes in the RhoA/ROCK signaling cascade are highly upregulated in the neurodegenerative microglia (MGnD) from APP/PS-1 transgenic Alzheimer's disease (AD) mice. In addition to illuminating the specific roles of RhoA/ROCK signaling in neuroinflammation, we demonstrate the utility of using IMG cells as a model for primary microglia in cellular studies.
Keywords: NF-κB; ROCK inhibitors; ROCK1; ROCK2; RhoA; microglia; neuroinflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

