Co-Stimulation of AGEs and LPS Induces Inflammatory Mediators through PLCγ1/JNK/NF-κB Pathway in MC3T3-E1 Cells
- PMID: 37408216
- PMCID: PMC10216316
- DOI: 10.3390/cells12101383
Co-Stimulation of AGEs and LPS Induces Inflammatory Mediators through PLCγ1/JNK/NF-κB Pathway in MC3T3-E1 Cells
Abstract
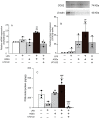
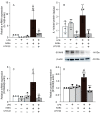
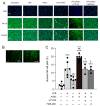
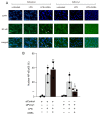
Advanced glycation end-products (AGEs) are increased under hyperglycemia in vivo and are associated with the onset of diabetes. According to previous studies, AGEs exacerbate inflammatory diseases. However, the mechanism by which AGEs aggravate osteoblast inflammation remains unknown. Therefore, the aim of this study was to determine the effects of AGEs on the production of inflammatory mediators in MC3T3-E1 cells and the underlying molecular mechanisms. Co-stimulation with AGEs and lipopolysaccharide (LPS) was found to increase the mRNA and protein levels of cyclooxygenase 2 (COX2), interleukin-1α (IL-1α), S100 calcium-binding protein A9 (S100A9), and the production of prostaglandin E2 (PGE2) compared to no stimulation (untreated control) or individual stimulation with LPS or AGEs. In contrast, the phospholipase C (PLC) inhibitor, U73122, inhibited these stimulatory effects. Co-stimulation with AGEs and LPS also increased the nuclear translocation of nuclear factor-kappa B (NF-κB) compared to no stimulation (untreated control) or individual stimulation with LPS or AGE. However, this increase was inhibited by U73122. Co-stimulation with AGEs and LPS-induced phosphorylated phospholipase Cγ1 (p-PLCγ1) and phosphorylated c-Jun N-terminal kinase (p-JNK) expression compared to no stimulation or individual stimulation with LPS or AGEs. U73122 inhibited the effects induced by co-stimulation. siPLCγ1 did not increase the expression of p-JNK and the translocation of NF-κB. Overall, co-stimulation with AGEs and LPS may promote inflammation mediators in MC3T3-E1 cells by activating the nuclear translocation of NF-κB via PLCγ1-JNK activation.
Keywords: AGEs; IL-1α; JNK; LPS; NF-κB; PGE2; PLCγ1; S100A9.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Miura J., Yamagishi S., Uchigata Y., Takeichi M., Yamamoto H., Makita Z., Iwamoto Y. Serum Levels of Non-carboxymethyllysine Advanced Glycation Endproducts are Correlated to Severity of Microvascular Complications in Patients with Type 1 Diabetes. J. Diabetes Complicat. 2003;17:16–21. doi: 10.1016/S1056-8727(02)00183-6. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

