Next-Generation Boron Drugs and Rational Translational Studies Driving the Revival of BNCT
- PMID: 37408232
- PMCID: PMC10216459
- DOI: 10.3390/cells12101398
Next-Generation Boron Drugs and Rational Translational Studies Driving the Revival of BNCT
Abstract
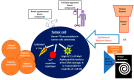
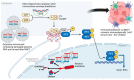
BNCT is a high-linear-energy transfer therapy that facilitates tumor-directed radiation delivery while largely sparing adjacent normal tissues through the biological targeting of boron compounds to tumor cells. Tumor-specific accumulation of boron with limited accretion in normal cells is the crux of successful BNCT delivery. Given this, developing novel boronated compounds with high selectivity, ease of delivery, and large boron payloads remains an area of active investigation. Furthermore, there is growing interest in exploring the immunogenic potential of BNCT. In this review, we discuss the basic radiobiological and physical aspects of BNCT, traditional and next-generation boron compounds, as well as translational studies exploring the clinical applicability of BNCT. Additionally, we delve into the immunomodulatory potential of BNCT in the era of novel boron agents and examine innovative avenues for exploiting the immunogenicity of BNCT to improve outcomes in difficult-to-treat malignancies.
Keywords: BNCT; High-LET; immunomodulation; novel boron compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Maliszewska-Olejniczak K., Kaniowski D., Araszkiewicz M., Tymińska K., Korgul A. Molecular Mechanisms of Specific Cellular DNA Damage Response and Repair Induced by the Mixed Radiation Field During Boron Neutron Capture Therapy. Front. Oncol. 2021;11:676575. doi: 10.3389/fonc.2021.676575. - DOI - PMC - PubMed
-
- Altieri S., Protti N. A Brief Review on Reactor-Based Neutron Sources for Boron Neutron Capture Therapy. Ther. Radiol. Oncol. 2018;2:47. doi: 10.21037/tro.2018.10.08. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

