Reduced metal nanocatalysts for selective electrochemical hydrogenation of biomass-derived 5-(hydroxymethyl)furfural to 2,5-bis(hydroxymethyl)furan in ambient conditions
- PMID: 37408562
- PMCID: PMC10318534
- DOI: 10.3389/fchem.2023.1200469
Reduced metal nanocatalysts for selective electrochemical hydrogenation of biomass-derived 5-(hydroxymethyl)furfural to 2,5-bis(hydroxymethyl)furan in ambient conditions
Abstract
Selective electrochemical hydrogenation (ECH) of biomass-derived unsaturated organic molecules has enormous potential for sustainable chemical production. However, an efficient catalyst is essential to perform an ECH reaction consisting of superior product selectivity and a higher conversion rate. Here, we examined the ECH performance of reduced metal nanostructures, i.e., reduced Ag (rAg) and reduced copper (rCu) prepared via electrochemical or thermal oxidation and electrochemical reduction process, respectively. Surface morphological analysis suggests the formation of nanocoral and entangled nanowire structure formation for rAg and rCu catalysts. rCu exhibits a slight enhancement in ECH reaction performance in comparison to the pristine Cu. However, the rAg exhibits more than two times higher ECH performance without compromising the selectivity for 5-(HydroxyMethyl) Furfural (HMF) to 2,5-bis(HydroxyMethyl)-Furan (BHMF) formation in comparison to the Ag film. Moreover, a similar ECH current density was recorded at a reduced working potential of 220 mV for rAg. This high performance of rAg is attributed to the formation of new catalytically active sites during the Ag oxidation and reduction processes. This study demonstrates that rAg can potentially be used for the ECH process with minimum energy consumption and a higher production rate.
Keywords: 2,5-bis(hydroxymethyl)furan (BHMF); 5-(hydroxymethyl)furfural (HMF); biomass; electrocatalysts; electrochemical hydrogenation; nanocoral Ag.
Copyright © 2023 Muchharla, Dikshit, Pokharel, Garimella, Adedeji, Kumar, Cao, Elsayed-Ali, Sadasivuni, Al-Dhabi, Kumar and Kumar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
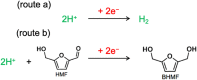

 AgCl (111),
AgCl (111),  AgCl (220) and
AgCl (220) and  AgCl (222)] and reduced Ag, (D) Cu after thermal oxidation at 400°C for 1 h (in the inset pristine Cu), (E) Cu after electrochemical reduction in 0.1 M KHCO3 solution at 0.3 V versus RHE. The scale bar is 5 µm for (A, B) and 2.5 µm for (D–F) XRD analysis of pristine Cu, oxidized Cu [
AgCl (222)] and reduced Ag, (D) Cu after thermal oxidation at 400°C for 1 h (in the inset pristine Cu), (E) Cu after electrochemical reduction in 0.1 M KHCO3 solution at 0.3 V versus RHE. The scale bar is 5 µm for (A, B) and 2.5 µm for (D–F) XRD analysis of pristine Cu, oxidized Cu [ Cu2O (110)
Cu2O (110)  Cu2O (111)
Cu2O (111)  Cu2O (200)
Cu2O (200)  Cu2O (220) and
Cu2O (220) and  Cu2O (311)] and reduced Cu.
Cu2O (311)] and reduced Cu.

References
-
- Bozell J. J., Petersen G. R. (2010). Technology development for the production of biobased products from biorefinery carbohydrates—The US department of energy’s “top 10” revisited. Green Chem. 12, 539–554. 10.1039/b922014c - DOI
-
- Chadderdon X. H., Chadderdon D. J., Pfennig T., Shanks B. H., Li W. (2019). Paired electrocatalytic hydrogenation and oxidation of 5-(hydroxymethyl)furfural for efficient production of biomass-derived monomers. Green Chem. 21, 6210–6219. 10.1039/c9gc02264c - DOI
-
- Chatterjee M., Ishizaka T., Kawanami H. (2014). Selective hydrogenation of 5-hydroxymethylfurfural to 2,5-bis-(hydroxymethyl)furan using Pt/MCM-41 in an aqueous medium: A simple approach. Green Chem. 16, 4734–4739. 10.1039/c4gc01127a - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

