In-depth genome and pan-genome analysis of a metal-resistant bacterium Pseudomonas parafulva OS-1
- PMID: 37408640
- PMCID: PMC10318148
- DOI: 10.3389/fmicb.2023.1140249
In-depth genome and pan-genome analysis of a metal-resistant bacterium Pseudomonas parafulva OS-1
Abstract
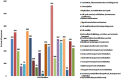
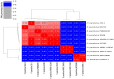
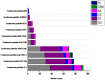
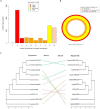
A metal-resistant bacterium Pseudomonas parafulva OS-1 was isolated from waste-contaminated soil in Ranchi City, India. The isolated strain OS-1 showed its growth at 25-45°C, pH 5.0-9.0, and in the presence of ZnSO4 (upto 5 mM). Phylogenetic analysis based on 16S rRNA gene sequences revealed that strain OS-1 belonged to the genus Pseudomonas and was most closely related to parafulva species. To unravel the genomic features, we sequenced the complete genome of P. parafulva OS-1 using Illumina HiSeq 4,000 sequencing platform. The results of average nucleotide identity (ANI) analysis indicated the closest similarity of OS-1 to P. parafulva PRS09-11288 and P. parafulva DTSP2. The metabolic potential of P. parafulva OS-1 based on Clusters of Othologous Genes (COG) and Kyoto Encyclopedia of Genes and Genomes (KEGG) indicated a high number of genes related to stress protection, metal resistance, and multiple drug-efflux, etc., which is relatively rare in P. parafulva strains. Compared with other parafulva strains, P. parafulva OS-1 was found to have the unique β-lactam resistance and type VI secretion system (T6SS) gene. Additionally, its genomes encode various CAZymes such as glycoside hydrolases and other genes associated with lignocellulose breakdown, suggesting that strain OS-1 have strong biomass degradation potential. The presence of genomic complexity in the OS-1 genome indicates that horizontal gene transfer (HGT) might happen during evolution. Therefore, genomic and comparative genome analysis of parafulva strains is valuable for further understanding the mechanism of resistance to metal stress and opens a perspective to exploit a newly isolated bacterium for biotechnological applications.
Keywords: Pseudomonas parafulva; carbohydrate active enzymes; genome; pan-genome; virulence.
Copyright © 2023 Kumari, Rawat, Shadan, Sharma, Deb and Singh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Archibald A. R., Hancock I. C., Harwood C. R. (1993). “Cell wall structure, synthesis, and turnover” in Bacillus subtilis and other gram-positive bacteria: Biochemistry, Physiology, and Molecular Genetics. eds. Sonenshein A. L., Hoch J. A., Losick R. (Hoboken, NJ: Wiley; ), 379–410.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

