Combining immunotherapy with high-dose radiation therapy (HDRT) significantly inhibits tumor growth in a syngeneic mouse model of high-risk neuroblastoma
- PMID: 37408891
- PMCID: PMC10319189
- DOI: 10.1016/j.heliyon.2023.e17399
Combining immunotherapy with high-dose radiation therapy (HDRT) significantly inhibits tumor growth in a syngeneic mouse model of high-risk neuroblastoma
Abstract
Purpose: The mortality in patients with MYCN-amplified high-risk neuroblastoma remains greater than 50% despite advances in multimodal therapy. Novel therapies are urgently needed that requires preclinical evaluation in appropriate mice models. Combinatorial treatment with high-dose radiotherapy (HDRT) and immunotherapy has emerged as an effective treatment option in a variety of cancers. Current models of neuroblastoma do not recapitulate the anatomic and immune environment in which multimodal therapies can be effectively tested, and there is a need for an appropriate syngeneic neuroblastoma mice model to study interaction of immunotherapy with host immune cells. Here, we develop a novel syngeneic mouse model of MYCN-amplified neuroblastoma and report the relevance and opportunities of this model to study radiotherapy and immunotherapy.
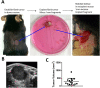
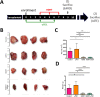
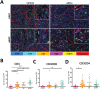
Materials and methods: A syngeneic allograft tumor model was developed using the murine neuroblastoma cell line 9464D derived a tumor from TH-MYCN transgenic mouse. Tumors were generated by transplanting 1 mm3 portions of 9464D flank tumors into the left kidney of C57Bl/6 mice. We investigated the effect of combining HDRT with anti-PD1 antibody on tumor growth and tumor microenvironment. HDRT (8 Gy x 3) was delivered by the small animal radiation research platform (SARRP). Tumor growth was monitored by ultrasound. To assess the effect on immune cells tumors sections were co-imuunostained for six biomarkers using the Vectra multispectral imaging platform.
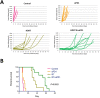
Results: Tumor growth was uniform and confined to the kidney in 100% of transplanted tumors. HDRT was largely restricted to the tumor region with minimal scattered out-of-field dose. Combinatorial treatment with HDRT and PD-1 blockade significantly inhibited tumor growth and prolonged mice survival. We observed augmented T-lymphocyte infiltration, especially CD3+CD8+ lymphocytes, in tumors of mice which received combination treatment.
Conclusion: We have developed a novel syngeneic mouse model of MYCN amplified high-risk neuroblastoma. We have utilized this model to show that combining immunotherapy with HDRT inhibits tumor growth and prolongs mice survival.
Keywords: Cancer therapeutics; Combination therapy; High dose radiation therapy; Immunotherapy; MYCN-amplified neuroblastoma; Neuroblastoma; Novel syngeneic mouse model.
© 2023 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
A transplantable TH-MYCN transgenic tumor model in C57Bl/6 mice for preclinical immunological studies in neuroblastoma.Int J Cancer. 2014 Mar 15;134(6):1335-45. doi: 10.1002/ijc.28463. Epub 2013 Sep 14. Int J Cancer. 2014. PMID: 24038106
-
Immune characterization of pre-clinical murine models of neuroblastoma.Sci Rep. 2020 Oct 7;10(1):16695. doi: 10.1038/s41598-020-73695-9. Sci Rep. 2020. PMID: 33028899 Free PMC article.
-
Combined innate and adaptive immunotherapy overcomes resistance of immunologically cold syngeneic murine neuroblastoma to checkpoint inhibition.J Immunother Cancer. 2019 Dec 6;7(1):344. doi: 10.1186/s40425-019-0823-6. J Immunother Cancer. 2019. PMID: 31810498 Free PMC article.
-
Application of individualized multimodal radiotherapy combined with immunotherapy in metastatic tumors.Front Immunol. 2023 Jan 12;13:1106644. doi: 10.3389/fimmu.2022.1106644. eCollection 2022. Front Immunol. 2023. PMID: 36713375 Free PMC article. Review.
-
Modulation of Radiation Doses and Chimeric Antigen Receptor T Cells: A Promising New Weapon in Solid Tumors-A Narrative Review.J Pers Med. 2023 Aug 14;13(8):1261. doi: 10.3390/jpm13081261. J Pers Med. 2023. PMID: 37623511 Free PMC article. Review.
Cited by
-
The neuroblastoma tumor microenvironment: From an in-depth characterization towards novel therapies.EJC Paediatr Oncol. 2024 Jun;3:100161. doi: 10.1016/j.ejcped.2024.100161. Epub 2024 Apr 7. EJC Paediatr Oncol. 2024. PMID: 39036648 Free PMC article.
-
Targeted delivery of napabucasin with radiotherapy improves outcomes in diffuse midline glioma.Neuro Oncol. 2025 Mar 7;27(3):795-810. doi: 10.1093/neuonc/noae215. Neuro Oncol. 2025. PMID: 39394920
-
Evaluation of a Combinatorial Immunotherapy Regimen That Can Cure Mice Bearing MYCN-Driven High-Risk Neuroblastoma That Resists Current Clinical Therapy.J Clin Med. 2024 Apr 26;13(9):2561. doi: 10.3390/jcm13092561. J Clin Med. 2024. PMID: 38731089 Free PMC article.
References
-
- Rha S.E., Byun J.Y., Jung S.E., Chun H.J., Lee H.G., Lee J.M. Neurogenic tumors in the abdomen: tumor types and imaging characteristics. Radiographics : Rev. Pub. Radiolog. Soci. North Am. Inc. 2003;23(1):29–43. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

