Imaging whole-brain activity to understand behavior
- PMID: 37409001
- PMCID: PMC10320740
- DOI: 10.1038/s42254-022-00430-w
Imaging whole-brain activity to understand behavior
Abstract
The brain evolved to produce behaviors that help an animal inhabit the natural world. During natural behaviors, the brain is engaged in many levels of activity from the detection of sensory inputs to decision-making to motor planning and execution. To date, most brain studies have focused on small numbers of neurons that interact in limited circuits. This allows analyzing individual computations or steps of neural processing. During behavior, however, brain activity must integrate multiple circuits in different brain regions. The activities of different brain regions are not isolated, but may be contingent on one another. Coordinated and concurrent activity within and across brain areas is organized by (1) sensory information from the environment, (2) the animal's internal behavioral state, and (3) recurrent networks of synaptic and non-synaptic connectivity. Whole-brain recording with cellular resolution provides a new opportunity to dissect the neural basis of behavior, but whole-brain activity is also mutually contingent on behavior itself. This is especially true for natural behaviors like navigation, mating, or hunting, which require dynamic interaction between the animal, its environment, and other animals. In such behaviors, the sensory experience of an unrestrained animal is actively shaped by its movements and decisions. Many of the signaling and feedback pathways that an animal uses to guide behavior only occur in freely moving animals. Recent technological advances have enabled whole-brain recording in small behaving animals including nematodes, flies, and zebrafish. These whole-brain experiments capture neural activity with cellular resolution spanning sensory, decision-making, and motor circuits, and thereby demand new theoretical approaches that integrate brain dynamics with behavioral dynamics. Here, we review the experimental and theoretical methods that are being employed to understand animal behavior and whole-brain activity, and the opportunities for physics to contribute to this emerging field of systems neuroscience.
Figures
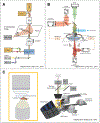
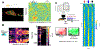
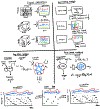
References
-
- Chen Jerry L., Andermann Mark L., Keck Tara, Xu Ning Long, and Ziv Yaniv. Imaging neuronal populations in behaving rodents: Paradigms for studying neural circuits underlying behavior in the mammalian cortex. J. Neurosci, 33(45):17631–17640, 2013. ISSN 02706474. doi: 10.1523/JNEUROSCI.3255-13.2013. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
