Design, synthesis and antitumor activity of Ascaphin-8 derived stapled peptides based on halogen-sulfhydryl click chemical reactions
- PMID: 37409042
- PMCID: PMC10318414
- DOI: 10.1039/d3ra02743k
Design, synthesis and antitumor activity of Ascaphin-8 derived stapled peptides based on halogen-sulfhydryl click chemical reactions
Abstract
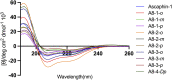
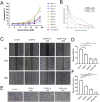
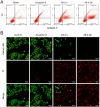
Ascaphin-8 (GFKDLLKGAAKALVKTVLF-NH2), isolated from the norepinephrine-stimulated skin secretion of the North American-tailed frog Ascaphus truei, is a C-terminal α-helical antimicrobial peptide with potential antitumor activity. However, linear peptides are difficult to be applied directly as drugs because of their inherent defects, such as low hydrolytic enzyme tolerance and poor structural stability. In this study, we designed and synthesized a series of stapled peptides based on Ascaphin-8 via thiol-halogen click chemistry. Most of the stapled peptide derivatives showed enhanced antitumor activity. Among them, A8-2-o and A8-4-Dp had the most improved structural stability, stronger hydrolytic enzyme tolerance and highest biological activity. This research may provide a reference for the stapled modification of other similar natural antimicrobial peptides.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
LinkOut - more resources
Full Text Sources

