Investigation of the efficacy and safety of retinal inactivation as a treatment for amblyopia in cats
- PMID: 37409104
- PMCID: PMC10319065
- DOI: 10.3389/fnins.2023.1167007
Investigation of the efficacy and safety of retinal inactivation as a treatment for amblyopia in cats
Abstract
Introduction: Deprivation of normal vision early in postnatal development elicits modifications of neural circuitry within the primary visual pathway that can cause a severe and intractable vision impairment (amblyopia). In cats, amblyopia is often modeled with monocular deprivation (MD), a procedure that involves temporarily closing the lids of one eye. Following long-term MD, brief inactivation of the dominant eye's retina can promote recovery from the anatomical and physiological effects of MD. In consideration of retinal inactivation as a viable treatment for amblyopia it is imperative to compare its efficacy against conventional therapy, as well as assess the safety of its administration.
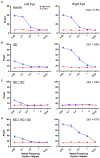
Methods: In the current study we compared the respective efficacies of retinal inactivation and occlusion of the dominant eye (reverse occlusion) to elicit physiological recovery from a prior long-term MD in cats. Because deprivation of form vision has been associated with development of myopia, we also examined whether ocular axial length or refractive error were altered by a period of retinal inactivation.
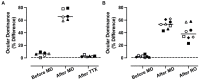
Results: The results of this study demonstrate that after a period of MD, inactivation of the dominant eye for up to 10 days elicited significant recovery of visually-evoked potentials that was superior to the recovery measured after a comparable duration of reverse occlusion. After monocular retinal inactivation, measurements of ocular axial length and refractive error were not significantly altered from their pre-inactivation values. The rate of body weight gain also was not changed during the period of inactivation, indicating that general well-being was not affected.
Discussion: These results provide evidence that inactivation of the dominant eye after a period of amblyogenic rearing promotes better recovery than eye occlusion, and this recovery was achieved without development of form-deprivation myopia.
Keywords: amblyopia; ocular axial length; plasticity; refractive error; retinal inactivation; tetrodotoxin; visual cortex; visually-evoked potentials.
Copyright © 2023 Hogan, DiCostanzo, Crowder, Fong and Duffy.
Conflict of interest statement
The authors declare that the research conducted for this study has no commercial or financial relationships that could be viewed as a conflict of interest.
Figures




References
-
- Birch E. E., Castañeda Y. S., Cheng-Patel C. S., Morale S. E., Kelly K. R., Beauchamp C. L., et al. (2019). Self-perception of school-aged children with amblyopia and its association with reading speed and motor skills. JAMA Ophthalmol. 137, 167–174. doi: 10.1001/jamaophthalmol.2018.5527, PMID: - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

