Second-line therapy for patients with steroid-refractory aGVHD: systematic review and meta-analysis of randomized controlled trials
- PMID: 37409129
- PMCID: PMC10318925
- DOI: 10.3389/fimmu.2023.1211171
Second-line therapy for patients with steroid-refractory aGVHD: systematic review and meta-analysis of randomized controlled trials
Abstract
Objective: Steroids-refractory (SR) acute graft-versus-host disease (aGVHD) is a life-threatening condition in patients undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT), but the optimal second-line therapy still has not been established. We aimed to perform a systematic review and meta-analysis of randomized controlled trials (RCTs) to compare the efficacy and safety of different second-line therapy regimens.
Methods: Literature search in MEDLINE, Embase, Cochrane Library and China Biology Medicine databases were performed to retrieve RCTs comparing the efficacy and safety of different therapy regimens for patients with SR aGVHD. Meta-analysis was conducted with Review Manager version 5.3. The primary outcome is the overall response rate (ORR) at day 28. Pooled relative risk (RR) and 95% confidence interval (CI) were calculated with the Mantel-Haenszel method.
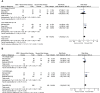
Results: Eight eligible RCTs were included, involving 1127 patients with SR aGVHD and a broad range of second-line therapy regimens. Meta-analysis of 3 trials investigating the effects of adding mesenchymal stroma cells (MSCs) to other second-line therapy regimens suggested that the addition of MSCs is associated with significantly improvement in ORR at day 28 (RR = 1.15, 95% CI = 1.01-1.32, P = 0.04), especially in patients with severe (grade III-IV or grade C-D) aGVHD (RR = 1.26, 95% CI = 1.04-1.52, P = 0.02) and patients with multiorgan involved (RR = 1.27, 95% CI = 1.05-1.55, P = 0.01). No significant difference was observed betwwen the MSCs group and control group in consideration of overall survival and serious adverse events. Treatment outcomes of the other trials were comprehensively reviewed, ruxolitinib showed significantly higher ORR and complete response rate at day 28, higher durable overall response at day 56 and longer failure-free survival in comparison with other regimens; inolimomab shows similar 1-year therapy success rate but superior long-term overall survial in comparison with anti-thymocyte globulin, other comparisons did not show significant differences in efficacy.
Conclusions: Adding MSCs to other second-line therapy regimens is associated with significantly improved ORR, ruxolitinib showed significantly better efficacy outcomes in comparison with other regimens in patients with SR aGVHD. Further well-designed RCTs and integrated studies are required to determine the optimal treatment.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022342487.
Keywords: acute graft-versus-host disease; mesenchymal stroma cells; meta-analysis; randomized controlled trials; ruxolitinib; second-line therapy; steroids-refractory.
Copyright © 2023 Luo, Huang, Wei, Wu, Huang, Ding, Huang, Chen, Li, Zou and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European society for blood and marrow transplantation. Lancet Haematol (2020) 7(2):e157–e67. doi: 10.1016/S2352-3026(19)30256-X - DOI - PubMed
-
- Cahn JY, Klein JP, Lee SJ, Milpied N, Blaise D, Antin JH, et al. Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: a joint société française de greffe de moëlle et thérapie cellulaire (SFGM-TC), Dana Farber cancer institute (DFCI), and international bone marrow transplant registry (IBMTR) prospective study. Blood (2005) 106(4):1495–500. doi: 10.1182/blood-2004-11-4557 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

