The hydrophilic extract from a new tomato genotype (named DHO) kills cancer cell lines through the modulation of the DNA damage response induced by Campthotecin treatment
- PMID: 37409248
- PMCID: PMC10318356
- DOI: 10.3389/fonc.2023.1117262
The hydrophilic extract from a new tomato genotype (named DHO) kills cancer cell lines through the modulation of the DNA damage response induced by Campthotecin treatment
Abstract
Introduction: DNA double-strand breaks are the most toxic lesions repaired through the non-homologous and joining (NHEJ) or the homologous recombination (HR), which is dependent on the generation of single-strand tails, by the DNA end resection mechanism. The resolution of the HR intermediates leads to error-free repair (Gene Conversion) or the mutagenic pathways (Single Strand Annealing and Alternative End-Joining); the regulation of processes leading to the resolution of the HR intermediates is not fully understood.
Methods: Here, we used a hydrophilic extract of a new tomato genotype (named DHO) in order to modulate the Camptothecin (CPT) DNA damage response.
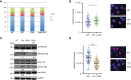
Results: We demonstrated increased phosphorylation of Replication Protein A 32 Serine 4/8 (RPA32 S4/8) protein in HeLa cells treated with the CPT in combination with DHO extract with respect to CPT alone. Moreover, we pointed out a change in HR intermediates resolution from Gene Conversion to Single Strand Annealing through the modified DNA repair protein RAD52 homolog (RAD52), DNA excision repair protein ERCC-1 (ERCC1) chromatin loading in response to DHO extract, and CPT co-treatment, with respect to the vehicle. Finally, we showed an increased sensitivity of HeLa cell lines to DHO extract and CPT co-treatment suggesting a possible mechanism for increasing the efficiency of cancer therapy.
Discussion: We described the potential role of DHO extract in the modulation of DNA repair, in response to Camptothecin treatment (CPT), favoring an increased sensitivity of HeLa cell lines to topoisomerase inhibitor therapy.
Keywords: DNA damage response; DNA repair; Solanum lycopersicum; homologous recombination; single strand annealing.
Copyright © 2023 Barone, Iannuzzi, Forte, Ragosta, Cuomo, Dell’Aquila, Altieri, Caporaso, Camerlingo, Rigano, Monti, Barone, Imbimbo, Frusciante, Monda, D’Angelo, De Laurentiis, Giordano and Alfano.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials

