Integrated physicochemical, hormonal, and transcriptomic analysis reveals the underlying mechanism of callus formation in Pinellia ternata hydroponic cuttings
- PMID: 37409296
- PMCID: PMC10319145
- DOI: 10.3389/fpls.2023.1189499
Integrated physicochemical, hormonal, and transcriptomic analysis reveals the underlying mechanism of callus formation in Pinellia ternata hydroponic cuttings
Abstract
Introduction: P. ternata is a perennial herb of the family Araceae that grows in China and has various medicinal properties and applications. At present, the artificial cultivation of P. ternata is constrained by seedling propagation. To address the problems of low seedling breeding propagation efficiency and high cost, our group has developed a highly efficient cultivation technology for "hydroponic cuttings of P. ternata "for the first time. P. ternata is used as the source material and is grown in a hydroponic system, increasing the seedling production rate 10-fold compared with the traditional cultivation mode. However, the callus formation mechanism in cuttings from hydroponic cultivation is still remains unclear.
Methods: In order to better understand the biological process of callus formation in cuttings from hydroponic P. ternata, anatomical characterization, endogenous hormone content determination and transcriptome sequencing were performed on five callus stages from early growth to early senescence.
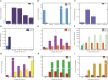
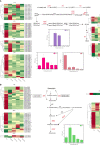
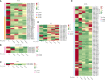
Results: Regarding the four major hormones during the callus developmental stages of P. ternata hydroponic cuttings, cytokinins showed an increasing trend during callus formation. IAA(indole-3-acetic acid) and abscisic acid contents increased at 8d and then decreased, while jasmonic acid content gradually decreased. A total of 254137 unigenes were identified by transcriptome sequencing in five callus formation stages. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of the differentially expressed genes (DEGs) that differentially expressed unigenes were involved in various plant hormone signaling and hormone synthesis-related pathways. The expression patterns of 7 genes were validated using quantitative real-time PCR.
Discussion: This study presented integrated transcriptomic and metabolic analysis approach to obtain insights into the underlying biosynthetic mechanisms and function of key hormones involved in the callus formation process from hydroponic P. ternata cuttings.
Keywords: P. ternate; histomorphological; phytohormone; plant hormone signaling and synthesis pathways; transcriptome.
Copyright © 2023 Duan, Chen, Liu, Chen, Wang and Hu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Ahuja M. R. (1965). Genetic control of tumor formation in higher plants. Q. Rev. Biol. 40, 329–340. doi: 10.1086/404744 - DOI
LinkOut - more resources
Full Text Sources

