Volume electron microscopy
- PMID: 37409324
- PMCID: PMC7614724
- DOI: 10.1038/s43586-022-00131-9
Volume electron microscopy
Abstract
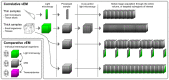
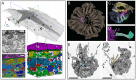
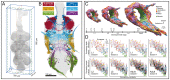
Life exists in three dimensions, but until the turn of the century most electron microscopy methods provided only 2D image data. Recently, electron microscopy techniques capable of delving deep into the structure of cells and tissues have emerged, collectively called volume electron microscopy (vEM). Developments in vEM have been dubbed a quiet revolution as the field evolved from established transmission and scanning electron microscopy techniques, so early publications largely focused on the bioscience applications rather than the underlying technological breakthroughs. However, with an explosion in the uptake of vEM across the biosciences and fast-paced advances in volume, resolution, throughput and ease of use, it is timely to introduce the field to new audiences. In this Primer, we introduce the different vEM imaging modalities, the specialized sample processing and image analysis pipelines that accompany each modality and the types of information revealed in the data. We showcase key applications in the biosciences where vEM has helped make breakthrough discoveries and consider limitations and future directions. We aim to show new users how vEM can support discovery science in their own research fields and inspire broader uptake of the technology, finally allowing its full adoption into mainstream biological imaging.
Conflict of interest statement
Competing Interests K.D.M. has founder’s equity interests in Aratome, LLC (Menlo Park, CA), an enterprise that produces array tomography materials and services. All other authors declare no competing interests.
Figures





References
-
- Peddie CJ, Schieber NL. In: Correlative Imaging: Focusing on the Future. Collinson Lucy, Verkade Paul., editors. John Wiley & Sons Ltd; 2020. pp. 37–66.
-
- Hayat MA. Principles and Techniques of electron microscopy: biological applications. Cambridge University Press; 2000. pp. 45–61.
Grants and funding
LinkOut - more resources
Full Text Sources
