Satellite liver transplant centres significantly improve transplant assessment outcomes for patients with chronic liver disease but not hepatocellular carcinoma: a retrospective cohort study
- PMID: 37409334
- PMCID: PMC11138172
- DOI: 10.1136/flgastro-2022-102366
Satellite liver transplant centres significantly improve transplant assessment outcomes for patients with chronic liver disease but not hepatocellular carcinoma: a retrospective cohort study
Abstract
Introduction: Liver transplantation (LT) remains integral to the management of end-stage chronic liver disease (CLD). However, referral thresholds and assessment pathways remain poorly defined. Distance from LT centre has been demonstrated to impact negatively on patient outcomes resulting in the development of satellite LT centres (SLTCs). We aimed to evaluate the impact of SLTCs on LT assessment in patients with CLD and hepatocellular carcinoma (HCC).
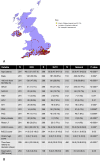
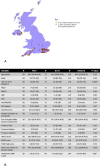
Methods: A retrospective cohort study was undertaken including all patients with CLD or HCC assessed for LT at King's College Hospital (KCH) between October 2014 and October 2019. Referral location, social, demographic, clinical and laboratory data were collected. Univariable and multivariable analyses (MVA) were performed to assess the impact of SLTCs on patients being accepted as LT candidates and contraindications being identified.
Results: 1102 and 240 LT assessments were included for patients with CLD and HCC, respectively. MVA demonstrated significant associations with; patients living greater than 60 min from KCH/SLTCs and LT candidacy acceptance in CLD, and less deprived patients and LT candidacy acceptance in HCC. However, neither variable was associated with identification of LT contraindications. MVA demonstrated that referrals from SLTCs were more likely to result in acceptance of LT candidacy and less likely to result in a contraindication being identified in CLD. However, such associations were not demonstrated in HCC.
Conclusion: SLTCs improve LT assessment outcomes in CLD but not HCC reflecting the standardised HCC referral pathway. Developing a formal regional LT assessment pathway across the UK would improve equity of access to transplantation.
Keywords: alcoholic liver disease; cirrhosis; health service research; hepatocellular carcinoma; liver transplantation.
© Author(s) (or their employer(s)) 2023. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- Williams R, Aspinall R, Bellis M, et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet 2014;384:1953–97. 10.1016/S0140-6736(14)61838-9 - DOI - PubMed
LinkOut - more resources
Full Text Sources
