Genetically Engineered-Cell-Membrane Nanovesicles for Cancer Immunotherapy
- PMID: 37409429
- PMCID: PMC10502869
- DOI: 10.1002/advs.202302131
Genetically Engineered-Cell-Membrane Nanovesicles for Cancer Immunotherapy
Abstract
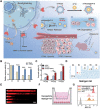
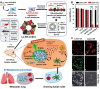
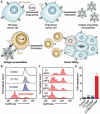
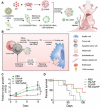
The advent of immunotherapy has marked a new era in cancer treatment, offering significant clinical benefits. Cell membrane as drug delivery materials has played a crucial role in enhancing cancer therapy because of their inherent biocompatibility and negligible immunogenicity. Different cell membranes are prepared into cell membrane nanovesicles (CMNs), but CMNs have limitations such as inefficient targeting ability, low efficacy, and unpredictable side effects. Genetic engineering has deepened the critical role of CMNs in cancer immunotherapy, enabling genetically engineered-CMN (GCMN)-based therapeutics. To date, CMNs that are surface modified by various functional proteins have been developed through genetic engineering. Herein, a brief overview of surface engineering strategies for CMNs and the features of various membrane sources is discussed, followed by a description of GCMN preparation methods. The application of GCMNs in cancer immunotherapy directed at different immune targets is addressed as are the challenges and prospects of GCMNs in clinical translation.
Keywords: cancer immunotherapy; cell membrane nanovesicles (CMNs); genetic engineering; genetically engineered-cell-membrane nanovesicles (GCMNs); immune targets.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













References
-
- Miller K. D., Siegel R. L., Lin C. C., Mariotto A. B., Kramer J. L., Rowland J. H., Stein K. D., Alteri R., Jemal A., Ca‐Cancer J. Clin. 2016, 66, 271. - PubMed
-
- a) Brahmer J. R., Tykodi S. S., Chow L. Q. M., Hwu W. J., Topalian S. L., Hwu P., Drake C. G., Camacho L. H., Kauh J., Odunsi K., Pitot H. C., Hamid O., Bhatia S., Martins R., Eaton K., Chen S. M., Salay T. M., Alaparthy S., Grosso J. F., Korman A. J., Parker S. M., Agrawal S., Goldberg S. M., Pardoll D. M., Gupta A., Wigginton J. M., N. Engl. J. Med. 2012, 366, 2455; - PMC - PubMed
- b) Zhao Z. Y., Dong S. M., Liu Y., Wang J. X., Ba L., Zhang C., Cao X. Y., Wu C. J., Yang P. P., ACS Nano 2022, 16, 20400; - PubMed
- c) Kraehenbuehl L., Weng C. H., Eghbali S., Wolchok J. D., Merghoub T., Nat. Rev. Clin. Oncol. 2022, 19, 37. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
