Extracellular Vesicle-Encapsulated Adeno-Associated Viruses for Therapeutic Gene Delivery to the Heart
- PMID: 37409482
- PMCID: PMC12462899
- DOI: 10.1161/CIRCULATIONAHA.122.063759
Extracellular Vesicle-Encapsulated Adeno-Associated Viruses for Therapeutic Gene Delivery to the Heart
Abstract
Background: Adeno-associated virus (AAV) has emerged as one of the best tools for cardiac gene delivery due to its cardiotropism, long-term expression, and safety. However, a significant challenge to its successful clinical use is preexisting neutralizing antibodies (NAbs), which bind to free AAVs, prevent efficient gene transduction, and reduce or negate therapeutic effects. Here we describe extracellular vesicle-encapsulated AAVs (EV-AAVs), secreted naturally by AAV-producing cells, as a superior cardiac gene delivery vector that delivers more genes and offers higher NAb resistance.
Methods: We developed a 2-step density-gradient ultracentrifugation method to isolate highly purified EV-AAVs. We compared the gene delivery and therapeutic efficacy of EV-AAVs with an equal titer of free AAVs in the presence of NAbs, both in vitro and in vivo. In addition, we investigated the mechanism of EV-AAV uptake in human left ventricular and human induced pluripotent stem cell-derived cardiomyocytes in vitro and mouse models in vivo using a combination of biochemical techniques, flow cytometry, and immunofluorescence imaging.
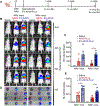
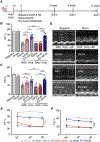
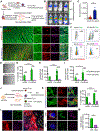
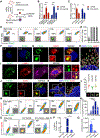
Results: Using cardiotropic AAV serotypes 6 and 9 and several reporter constructs, we demonstrated that EV-AAVs deliver significantly higher quantities of genes than AAVs in the presence of NAbs, both to human left ventricular and human induced pluripotent stem cell-derived cardiomyocytes in vitro and to mouse hearts in vivo. Intramyocardial delivery of EV-AAV9-sarcoplasmic reticulum calcium ATPase 2a to infarcted hearts in preimmunized mice significantly improved ejection fraction and fractional shortening compared with AAV9-sarcoplasmic reticulum calcium ATPase 2a delivery. These data validated NAb evasion by and therapeutic efficacy of EV-AAV9 vectors. Trafficking studies using human induced pluripotent stem cell-derived cells in vitro and mouse hearts in vivo showed significantly higher expression of EV-AAV6/9-delivered genes in cardiomyocytes compared with noncardiomyocytes, even with comparable cellular uptake. Using cellular subfraction analyses and pH-sensitive dyes, we discovered that EV-AAVs were internalized into acidic endosomal compartments of cardiomyocytes for releasing and acidifying AAVs for their nuclear uptake.
Conclusions: Together, using 5 different in vitro and in vivo model systems, we demonstrate significantly higher potency and therapeutic efficacy of EV-AAV vectors compared with free AAVs in the presence of NAbs. These results establish the potential of EV-AAV vectors as a gene delivery tool to treat heart failure.
Keywords: extracellular vesicles; genetic therapy.
Conflict of interest statement
Figures




References
-
- MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, Clark KR, During MJ, Cremers FP, Black GC, et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase ½ clinical trial. Lancet. 2014;383:1129–1137. doi: 10.1016/S0140-6736(13)62117-0 - DOI - PMC - PubMed
-
- Greenberg B, Butler J, Felker GM, Ponikowski P, Voors AA, Desai AS, Barnard D, Bouchard A, Jaski B, Lyon AR, et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet. 2016;387:1178–1186. doi: 10.1016/S0140-6736(16)00082-9 - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

