Long-term efficacy and safety of low-sodium oxybate in an open-label extension period of a placebo-controlled, double-blind, randomized withdrawal study in adults with idiopathic hypersomnia
- PMID: 37409509
- PMCID: PMC10545992
- DOI: 10.5664/jcsm.10698
Long-term efficacy and safety of low-sodium oxybate in an open-label extension period of a placebo-controlled, double-blind, randomized withdrawal study in adults with idiopathic hypersomnia
Abstract
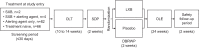
Study objectives: To evaluate 6-month efficacy and safety of low-sodium oxybate in people with idiopathic hypersomnia during an open-label extension period (OLE) of a phase 3 clinical trial.
Methods: Efficacy measures included the Epworth Sleepiness Scale (ESS), Idiopathic Hypersomnia Severity Scale (IHSS), Patient Global Impression of Change (PGIc), Functional Outcomes of Sleep Questionnaire, short version (FOSQ-10), and Work Productivity and Activity Impairment Questionnaire: Specific Health Problem (WPAI:SHP). Treatment-emergent adverse events were collected throughout the OLE.
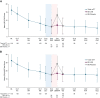
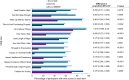
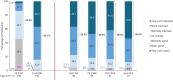
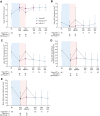
Results: The OLE population included 106 participants. Most were female (71%) and White (83%), and the mean (SD) age was 41.0 (13.8) years. ESS scores decreased (improved) during the OLE (mean [SD], study baseline: 16.3 [2.8]; OLE week 2: 6.7 [4.7]; OLE end: 5.3 [3.7]), and IHSS total scores trended toward a decrease (study baseline: 32.6 [7.3]; OLE week 2: 16.2 [8.9]; OLE end: 14.8 [8.6]. Median (minimum, maximum) paired differences from OLE week 2 to OLE end were ESS, -1.0 (-20, 7; nominal P = .012); IHSS, -1.0 (-31, 19; nominal P = .086). The proportion of participants reporting PGIc ratings of "very much improved" increased from 36.7% at OLE week 2 to 53.8% at the OLE end. The FOSQ-10 and WPAI:SHP scores remained stable during OLE. The incidence of newly reported treatment-emergent adverse events decreased over the duration of the OLE.
Conclusions: Efficacy and safety of low-sodium oxybate were maintained or improved during the 6-month OLE, supporting long-term treatment with low-sodium oxybate in adults with idiopathic hypersomnia.
Clinical trial registration: Registry: ClinicalTrials.gov; Name: A Multicenter Study of the Efficacy and Safety of JZP-258 in the Treatment of Idiopathic Hypersomnia (IH) With an Open-label Safety Extension; URL: https://clinicaltrials.gov/study/NCT03533114; Identifier: NCT03533114 and Registry: EU Clinical Trials; Name: A Double-blind, Placebo-controlled, Randomized Withdrawal, Multicenter Study of the Efficacy and Safety of JZP-258 in the Treatment of Idiopathic Hypersomnia (IH) with an Open-label Safety Extension; URL: https://www.clinicaltrialsregister.eu/ctr-search/trial/2018-001311-79/results; Identifier: 2018-001311-79.
Citation: Morse AM, Dauvilliers Y, Arnulf I, et al. Long-term efficacy and safety of low-sodium oxybate in an open-label extension period of a placebo-controlled, double-blind, randomized withdrawal study in adults with idiopathic hypersomnia. J Clin Sleep Med. 2023;19(10):1811-1822.
Keywords: quality of life; sleep inertia; sleepiness; work productivity.
© 2023 American Academy of Sleep Medicine.
Conflict of interest statement
All authors have seen and approved the manuscript. Work for this study was conducted at 50 specialist sleep centers in Belgium, Czech Republic, Finland, France, Poland, Spain, and the United States. This study was sponsored by Jazz Pharmaceuticals. Anne Marie Morse has received research/grant support and consultancy fees from Avadel, Harmony Biosciences, Jazz Pharmaceuticals, Takeda Pharmaceutical Co, Ltd, NLS Pharmaceutics, Alkermes, UCB Pharmaceuticals, and the National Institutes of Health (NIH). Yves Dauvilliers is a consultant for and has participated in advisory boards for Jazz Pharmaceuticals, UCB Pharma, Avadel, Harmony Biosciences, Idorsia, Orexia, Takeda, Paladin, and Bioprojet. Isabelle Arnulf has participated in advisory boards for Idorsia (2020), Ono (2019) Pharma, and Roche Pharma (2018). Michael J. Thorpy has received research/grant support and consultancy fees from Axsome, Balance Therapeutics, Flamel/Avadel, Harmony Biosciences, Jazz Pharmaceuticals, Suven Life Sciences Ltd, Takeda Pharmaceutical Co, Ltd, NLS Pharmaceutics, XW Pharma, Idorsia Pharmaceuticals, and Eisai Pharmaceuticals. Nancy Foldvary-Schaefer has served on an advisory committee for Jazz Pharmaceuticals and participated in clinical trials supported by Jazz Pharmaceuticals, Suven Life Sciences, and Takeda Pharmaceuticals. Patricia Chandler, Abby Chen, and Luke Hickey are full-time employees of Jazz Pharmaceuticals who, in the course of this employment, have received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals, plc. Jed Black is a part-time employee of Jazz Pharmaceuticals and shareholder of Jazz Pharmaceuticals plc. Richard K. Bogan is a shareholder of Watermark Medical and Healthy Humming, LLC; serves on the Board of Directors for Watermark; is a medical consultant to Jazz Pharmaceuticals, Harmony Biosciences, Avadel Pharmaceuticals, Takeda, and Oventus; has conducted industry-funded research for Avadel, Axsome, Bresotec, Bayer, Idorsia, Suven, Jazz Pharmaceuticals, Balance, NLS Pharmaceutics, Vanda, Merck, Eisai, Philips, Fresca, Takeda, LivaNova, Roche, Sanofi, Sommetrics, and Noctrix; and is on speaker’s bureaus for Jazz Pharmaceuticals, Eisai, and Harmony.
Figures

Comment in
-
Idiopathic hypersomnia: the long journey from classification to an efficacious drug.J Clin Sleep Med. 2023 Oct 1;19(10):1709-1710. doi: 10.5664/jcsm.10776. J Clin Sleep Med. 2023. PMID: 37555598 Free PMC article. No abstract available.
References
-
- Dauvilliers Y , Bogan RK , Arnulf I , Scammell TE , St Louis EK , Thorpy MJ . Clinical considerations for the diagnosis of idiopathic hypersomnia . Sleep Med Rev. 2022. ; 66 : 101709 . - PubMed
-
- American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed . Darien, IL: : American Academy of Sleep Medicine; ; 2014. .
-
- Bassetti C , Aldrich MS . Idiopathic hypersomnia. A series of 42 patients . Brain. 1997. ; 120 ( Pt 8 ): 1423 – 1435 . - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

