Compliance to genomic test recommendations to guide adjuvant chemotherapy decision-making in the case of hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer, in real-life settings
- PMID: 37409516
- PMCID: PMC10501273
- DOI: 10.1002/cam4.6315
Compliance to genomic test recommendations to guide adjuvant chemotherapy decision-making in the case of hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer, in real-life settings
Abstract
Background: Genomic tests are a useful tool for adjuvant chemotherapy decision-making in the case of hormone receptor-positive (HR+), and human epidermal growth factor receptor 2-negative (HER2-) breast cancer with intermediate prognostic factors. Real-life data on the use of tests can help identify the target population for testing.
Methods: French multicentric study (8 centers) including patients, all candidates for adjuvant chemotherapy for HR-positive, HER2-negative early breast cancer. We describe the percentage of tests performed outside recommendations, according to the year of testing. We calculated a ratio defined as the number of tests required to avoid chemotherapy for one patient, and according to patient and cancer characteristics. We then performed a cost-saving analysis using medical cost data over a period of 1 year from diagnosis, calculated from a previous study. Finally, we calculated the threshold of the ratio (number of tests required to avoid chemotherapy for one patient) below which the use of genomic tests was cost-saving.
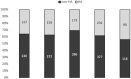
Results: A total of 2331 patients underwent a Prosigna test. The ratio (performed test/avoided chemotherapy) was 2.8 [95% CI: 2.7-2.9] in the whole population. In the group following recommendations for test indication, the ratio was 2.3 [95% CI: 2.2-2.4]. In the case of non-abidance by recommendations, the ratio was 3 [95% CI: 2.8-3.2]. Chemotherapy was avoided in 841 patients (36%) following the results of the Prosigna test. The direct medical costs saved over 1 year of care were 3,878,798€ and 1,718,472€ in the group of patients following test recommendations. We calculated that the ratio (performed test/avoided chemotherapy) needed to be under 6.9 for testing to prove cost-saving.
Conclusion: The use of genomic testing proved cost-saving in this large multicentric real-life analysis, even in certain cases when the test was performed outside recommendations.
Keywords: breast cancer; deescalation; genomic tests; guidelines.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures

References
-
- Cardoso F, van't Veer LJ, Bogaerts J, et al. 70‐gene signature as an aid to treatment decisions in early‐stage breast cancer. N Engl J Med. 2016;375(8):717‐729. - PubMed
-
- NCCN Guidelines Version 4 . Invasive Breast Cancer. 2022. Accessed Februeary 11, 2022. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
-
- Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO clinical practice guidelines. Ann Oncol. 2019;30:1194‐1220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

