Ergosterol distribution controls surface structure formation and fungal pathogenicity
- PMID: 37409809
- PMCID: PMC10470819
- DOI: 10.1128/mbio.01353-23
Ergosterol distribution controls surface structure formation and fungal pathogenicity
Erratum in
-
Correction for Choy et al., "Ergosterol distribution controls surface structure formation and fungal pathogenicity".mBio. 2025 Feb 5;16(2):e0355024. doi: 10.1128/mbio.03550-24. Epub 2024 Dec 17. mBio. 2025. PMID: 39688417 Free PMC article. No abstract available.
Abstract
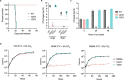
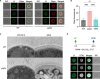
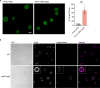
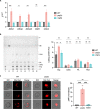
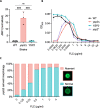
Ergosterol, the major sterol in fungal membranes, is critical for defining membrane fluidity and regulating cellular processes. Although ergosterol synthesis has been well defined in model yeast, little is known about sterol organization in the context of fungal pathogenesis. We identified a retrograde sterol transporter, Ysp2, in the opportunistic fungal pathogen Cryptococcus neoformans. We found that the lack of Ysp2 under host-mimicking conditions leads to abnormal accumulation of ergosterol at the plasma membrane, invagination of the plasma membrane, and malformation of the cell wall, which can be functionally rescued by inhibiting ergosterol synthesis with the antifungal drug fluconazole. We also observed that cells lacking Ysp2 mislocalize the cell surface protein Pma1 and have abnormally thin and permeable capsules. As a result of perturbed ergosterol distribution and its consequences, ysp2∆ cells cannot survive in physiologically relevant environments such as host phagocytes and are dramatically attenuated in virulence. These findings expand our knowledge of cryptococcal biology and underscore the importance of sterol homeostasis in fungal pathogenesis. IMPORTANCE Cryptococcus neoformans is an opportunistic fungal pathogen that kills over 100,000 people worldwide each year. Only three drugs are available to treat cryptococcosis, and these are variously limited by toxicity, availability, cost, and resistance. Ergosterol is the most abundant sterol in fungi and a key component in modulating membrane behavior. Two of the drugs used for cryptococcal infection, amphotericin B and fluconazole, target this lipid and its synthesis, highlighting its importance as a therapeutic target. We discovered a cryptococcal ergosterol transporter, Ysp2, and demonstrated its key roles in multiple aspects of cryptococcal biology and pathogenesis. These studies demonstrate the role of ergosterol homeostasis in C. neoformans virulence, deepen our understanding of a pathway with proven therapeutic importance, and open a new area of study.
Keywords: Cryptococcus neoformans; Ysp2; ergosterol; mycology; sterol transport; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Update of
-
Ergosterol distribution controls surface structure formation and fungal pathogenicity.bioRxiv [Preprint]. 2023 Feb 17:2023.02.17.528979. doi: 10.1101/2023.02.17.528979. bioRxiv. 2023. Update in: mBio. 2023 Aug 31;14(4):e0135323. doi: 10.1128/mbio.01353-23. PMID: 36824733 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

