Blockade-of-Binding Activities toward Envelope-Associated, Type-Specific Epitopes as a Correlative Marker for Dengue Virus-Neutralizing Antibody
- PMID: 37409936
- PMCID: PMC10433959
- DOI: 10.1128/spectrum.00918-23
Blockade-of-Binding Activities toward Envelope-Associated, Type-Specific Epitopes as a Correlative Marker for Dengue Virus-Neutralizing Antibody
Abstract
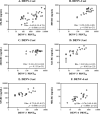
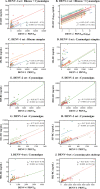
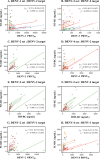
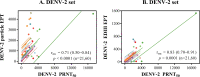
Humans infected with dengue virus (DENV) acquire long-term protection against the infecting serotype, whereas cross-protection against other serotypes is short-lived. Long-term protection induced by low levels of type-specific neutralizing antibodies can be assessed using the virus-neutralizing antibody test. However, this test is laborious and time-consuming. In this study, a blockade-of-binding enzyme-linked immunoassay was developed to assess antibody activity by using a set of neutralizing anti-E monoclonal antibodies and blood samples from dengue virus-infected or -immunized macaques. Diluted blood samples were incubated with plate-bound dengue virus particles before the addition of an enzyme-conjugated antibody specific to the epitope of interest. Based on blocking reference curves constructed using autologous purified antibodies, sample blocking activity was determined as the relative concentration of unconjugated antibody that resulted in the same percent signal reduction. In separate DENV-1-, -2-, -3-, and -4-related sets of samples, moderate to strong correlations of the blocking activity with neutralizing antibody titers were found with the four type-specific antibodies 1F4, 3H5, 8A1, and 5H2, respectively. Significant correlations were observed for single samples taken 1 month after infection as well as samples drawn before and at various time points after infection/immunization. Similar testing using a cross-reactive EDE-1 antibody revealed a moderate correlation between the blocking activity and the neutralizing antibody titer only for the DENV-2-related set. The potential usefulness of the blockade-of-binding activity as a correlative marker of neutralizing antibodies against dengue viruses needs to be validated in humans. IMPORTANCE This study describes a blockade-of-binding assay for the determination of antibodies that recognize a selected set of serotype-specific or group-reactive epitopes in the envelope of dengue virus. By employing blood samples collected from dengue virus-infected or -immunized macaques, moderate to strong correlations of the epitope-blocking activities with the virus-neutralizing antibody titers were observed with serotype-specific blocking activities for each of the four dengue serotypes. This simple, rapid, and less laborious method should be useful for the evaluation of antibody responses to dengue virus infection and may serve as, or be a component of, an in vitro correlate of protection against dengue in the future.
Keywords: ELISA; blockade of binding; dengue virus; neutralizing antibody.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- World Health Organization. 2011. Comprehensive guidelines for prevention and control of dengue and dengue hemorrhagic fever. Revised and expanded edition. World Health Organization Regional Office for Southeast Asia, New Delhi, India.
-
- Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, Brady OJ, Hay SI, Bedi N, Bensenor IM, Castañeda-Orjuela CA, Chuang T-W, Gibney KB, Memish ZA, Rafay A, Ukwaja KN, Yonemoto N, Murray CJL. 2016. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis 16:712–723. doi:10.1016/S1473-3099(16)00026-8. - DOI - PMC - PubMed
-
- Salje H, Alera MT, Chua MN, Hunsawong T, Ellison D, Srikiatkhachorn A, Jarman RG, Gromowski GD, Rodriguez-Barraquer I, Cauchemez S, Cummings DAT, Macareo L, Yoon I-K, Fernandez S, Rothman AL. 2021. Evaluation of the extended efficacy of the Dengvaxia vaccine against symptomatic and subclinical dengue infection. Nat Med 27:1395–1400. doi:10.1038/s41591-021-01392-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

