Incremental Utility of First-Pass Perfusion CMR for Prognostic Risk Stratification of Cancer-Associated Cardiac Masses
- PMID: 37410010
- PMCID: PMC11783222
- DOI: 10.1016/j.jcmg.2023.05.007
Incremental Utility of First-Pass Perfusion CMR for Prognostic Risk Stratification of Cancer-Associated Cardiac Masses
Abstract
Background: Cardiac magnetic resonance (CMR) differentiates cardiac metastasis (CMET) and cardiac thrombus (CTHR) based on tissue characteristics stemming from vascularity on late gadolinium enhancement (LGE). Perfusion CMR can assess magnitude of vascularity; utility for cardiac masses (CMASS) is unknown.
Objectives: This study sought to determine if perfusion CMR provides diagnostic and prognostic utility for CMASS beyond binary differentiation of CMET and CTHR.
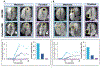
Methods: The population comprised adult cancer patients with CMASS on CMR; CMET and CTHR were defined using LGE-CMR: CMASS+ patients were matched to CMASS- control subjects for cancer type/stage. First-pass perfusion CMR was interpreted visually and semiquantitatively for CMASS vascularity, including contrast enhancement ratio (CER) (plateau vs baseline) and contrast uptake rate (CUR) (slope). Follow-up was performed for all-cause mortality.
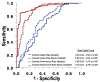
Results: A total of 462 cancer patients were studied, including patients with (CMET = 173, CTHR = 69) and without CMASS on LGE-CMR. On perfusion CMR, CER and CUR were higher within CMET vs CTHR (P < 0.001); CUR yielded better performance (AUC: 0.89-0.93) than CER (AUC: 0.66-0.72) (both P < 0.001) to differentiate LGE-CMR-evidenced CMET and CTHR, although both CUR (P = 0.10) and CER (P = 0.01) typically misclassified CMET with minimal enhancement. During follow-up, mortality among CMET patients was high but variable; 47% of patients were alive 1 year post-CMR. Patients with semiquantitative perfusion CMR-evidenced CMET had higher mortality than control subjects (HR: 1.42 [95% CI: 1.06-1.90]; P = 0.02), paralleling visual perfusion CMR (HR: 1.47 [95% CI: 1.12-1.94]; P = 0.006) and LGE-CMR (HR: 1.52 [95% CI: 1.16-2.00]; P = 0.003). Among patients with CMET on LGE-CMR, mortality was highest among patients (P = 0.002) with lesions in the bottom perfusion (CER) tertile, corresponding to low vascularity. Among CMET and cancer-matched control subjects, mortality was equivalent (P = NS) among patients with lesions in the upper CER tertile (corresponding to higher lesion vascularity). Conversely, patients with CMET in the middle (P = 0.03) and lowest (lowest vascularity) (P = 0.001) CER tertiles had increased mortality.
Conclusions: Perfusion CMR yields prognostic utility that complements LGE-CMR: Among cancer patients with LGE-CMR defined CMET, mortality increases in proportion to magnitude of lesion hypoperfusion.
Keywords: cardiac magnetic resonance; cardiac masses; cardio-oncology; late gadolinium enhancement; perfusion.
Copyright © 2024 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This work was supported by the National Institutes of Health 1R01HL151686 (to Dr Weinsaft), AHA18CDA34080090 and NYS DOH01-ROWLY6 2021- 00048 (to Dr Chan), and MSK SKI Core Grant P30 CA008748. Dr Chan holds a secondary appointment in the Mount Sinai Department of Pharmacology for basic science research unrelated to this study. Dr Weinsaft has received speaker fees from GE Healthcare for talks on cardiac magnetic resonance imaging; and serves as a consultant for Bitterroot Bio. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

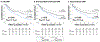
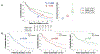

Comment in
-
First-Pass Perfusion Cardiac Magnetic Resonance Imaging for Cancer-Associated Cardiac Masses: First Impressions Count!JACC Cardiovasc Imaging. 2024 Feb;17(2):146-148. doi: 10.1016/j.jcmg.2023.06.020. Epub 2023 Aug 16. JACC Cardiovasc Imaging. 2024. PMID: 37589606 No abstract available.
References
-
- Sung H, Ferlay J, Siegel RL et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209–249. - PubMed
-
- Weinsaft JW, Kim HW, Shah DJ et al. Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol 2008;52:148–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

