Health Outcomes and Cost-effectiveness of Monoclonal SARS-CoV-2 Antibodies as Pre-exposure Prophylaxis
- PMID: 37410460
- PMCID: PMC10326646
- DOI: 10.1001/jamanetworkopen.2023.21985
Health Outcomes and Cost-effectiveness of Monoclonal SARS-CoV-2 Antibodies as Pre-exposure Prophylaxis
Abstract
Importance: Pre-exposure prophylaxis with neutralizing SARS-CoV-2 monoclonal antibodies (mAbs PrEP) prevents infection and reduces hospitalizations and the duration thereof for COVID-19 and death among high-risk individuals. However, reduced effectiveness due to a changing SARS-CoV-2 viral landscape and high drug prices remain substantial implementation barriers.
Objective: To assess the cost-effectiveness of mAbs PrEP as COVID-19 PrEP.
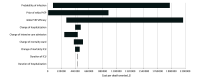
Design, setting, and participants: For this economic evaluation, a decision analytic model was developed and parameterized with health care outcome and utilization data from individuals with high risk for COVID-19. The SARS-CoV-2 infection probability, mAbs PrEP effectiveness, and drug pricing were varied. All costs were collected from a third-party payer perspective. Data were analyzed from September 2021 to December 2022.
Main outcomes and measures: Health care outcomes including new SARS-CoV-2 infections, hospitalization, and deaths. The cost per death averted and cost-effectiveness ratios using a threshold for prevention interventions of $22 000 or less per quality-adjusted life year (QALY) gained.
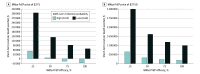
Results: The clinical cohort consisted of 636 individuals with COVID-19 (mean [SD] age 63 [18] years; 341 [54%] male). Most individuals were at high risk for severe COVID-19, including 137 (21%) with a body mass index of 30 or higher, 60 (9.4%) with hematological malignant neoplasm, 108 (17%) post-transplantation, and 152 (23.9%) who used immunosuppressive medication before COVID-19. Within the context of a high (18%) SARS-CoV-2 infection probability and low (25%) effectiveness the model calculated a short-term reduction of 42% ward admissions, 31% intensive care unit (ICU) admissions, and 34% deaths. Cost-saving scenarios were obtained with drug prices of $275 and 75% or higher effectiveness. With a 100% effectiveness mAbs PrEP can reduce ward admissions by 70%, ICU admissions by 97%, and deaths by 92%. Drug prices, however, need to reduce to $550 for cost-effectiveness ratios less than $22 000 per QALY gained per death averted and to $2200 for ratios between $22 000 and $88 000.
Conclusions and relevance: In this study, use of mAbs PrEP for preventing SARS-CoV-2 infections was cost-saving at the beginning of an epidemic wave (high infection probability) with 75% or higher effectiveness and drug price of $275. These results are timely and relevant for decision-makers involved in mAbs PrEP implementation. When newer mAbs PrEP combinations become available, guidance on implementation should be formulated ensuring a fast rollout. Nevertheless, advocacy for mAbs PrEP use and critical discussion on drug prices are necessary to ensuring cost-effectiveness for different epidemic settings.
Conflict of interest statement
Figures

References
-
- Nyberg T, Ferguson NM, Nash SG, et al. ; COVID-19 Genomics UK (COG-UK) consortium . Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303-1312. doi: 10.1016/S0140-6736(22)00462-7 - DOI - PMC - PubMed
-
- Haggenburg S, Hofsink Q, Lissenberg-Witte BI, et al. ; COBRA KAI Study Team . Antibody response in immunocompromised patients with hematologic cancers who received a 3-dose mRNA-1273 vaccination schedule for COVID-19. JAMA Oncol. 2022;8(10):1477-1483. doi: 10.1001/jamaoncol.2022.3227 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

