Fifty years of gating currents and channel gating
- PMID: 37410612
- PMCID: PMC10324510
- DOI: 10.1085/jgp.202313380
Fifty years of gating currents and channel gating
Abstract
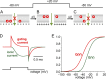
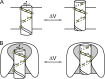
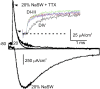
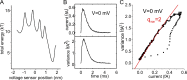
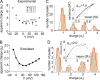
We celebrate this year the 50th anniversary of the first electrophysiological recordings of the gating currents from voltage-dependent ion channels done in 1973. This retrospective tries to illustrate the context knowledge on channel gating and the impact gating-current recording had then, and how it continued to clarify concepts, elaborate new ideas, and steer the scientific debate in these 50 years. The notion of gating particles and gating currents was first put forward by Hodgkin and Huxley in 1952 as a necessary assumption for interpreting the voltage dependence of the Na and K conductances of the action potential. 20 years later, gating currents were actually recorded, and over the following decades have represented the most direct means of tracing the movement of the gating charges and gaining insights into the mechanisms of channel gating. Most work in the early years was focused on the gating currents from the Na and K channels as found in the squid giant axon. With channel cloning and expression on heterologous systems, other channels as well as voltage-dependent enzymes were investigated. Other approaches were also introduced (cysteine mutagenesis and labeling, site-directed fluorometry, cryo-EM crystallography, and molecular dynamics [MD] modeling) to provide an integrated and coherent view of voltage-dependent gating in biological macromolecules. The layout of this retrospective reflects the past 50 years of investigations on gating currents, first addressing studies done on Na and K channels and then on other voltage-gated channels and non-channel structures. The review closes with a brief overview of how the gating-charge/voltage-sensor movements are translated into pore opening and the pathologies associated with mutations targeting the structures involved with the gating currents.
© 2023 Catacuzzeno et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures

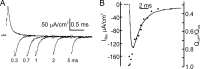
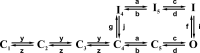
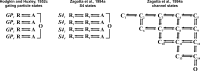

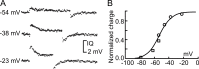

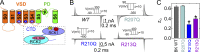
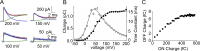
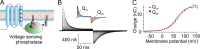
Similar articles
-
The 70-year search for the voltage-sensing mechanism of ion channels.J Physiol. 2022 Jul;600(14):3227-3247. doi: 10.1113/JP282780. Epub 2022 Jul 6. J Physiol. 2022. PMID: 35665931 Free PMC article.
-
Gating charge displacement in a monomeric voltage-gated proton (Hv1) channel.Proc Natl Acad Sci U S A. 2018 Sep 11;115(37):9240-9245. doi: 10.1073/pnas.1809705115. Epub 2018 Aug 20. Proc Natl Acad Sci U S A. 2018. PMID: 30127012 Free PMC article.
-
Coupling between charge movement and pore opening in voltage dependent potassium channels.Medicina (B Aires). 1995;55(5 Pt 2):591-9. Medicina (B Aires). 1995. PMID: 8842189
-
The voltage sensor in voltage-dependent ion channels.Physiol Rev. 2000 Apr;80(2):555-92. doi: 10.1152/physrev.2000.80.2.555. Physiol Rev. 2000. PMID: 10747201 Review.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
Cited by
-
The Long Journey from Animal Electricity to the Discovery of Ion Channels and the Modelling of the Human Brain.Biomolecules. 2024 Jun 12;14(6):684. doi: 10.3390/biom14060684. Biomolecules. 2024. PMID: 38927086 Free PMC article. Review.
-
Mitochondrial sAC-cAMP-PKA Axis Modulates the ΔΨm-Dependent Control Coefficients of the Respiratory Chain Complexes: Evidence of Respirasome Plasticity.Int J Mol Sci. 2023 Oct 13;24(20):15144. doi: 10.3390/ijms242015144. Int J Mol Sci. 2023. PMID: 37894823 Free PMC article.
-
The crucial decade that ion channels were proven to exist : The vision of Bertil Hille and Clay Armstrong and how it came through.Pflugers Arch. 2025 Jul;477(7):903-917. doi: 10.1007/s00424-025-03085-5. Epub 2025 Apr 22. Pflugers Arch. 2025. PMID: 40261416 Free PMC article. Review.
-
Inactivation of CaV1 and CaV2 channels.J Gen Physiol. 2025 Mar 3;157(2):e202313531. doi: 10.1085/jgp.202313531. Epub 2025 Jan 30. J Gen Physiol. 2025. PMID: 39883005 Review.
-
Distinct mechanisms of inhibition of Kv2 potassium channels by tetraethylammonium and RY785.bioRxiv [Preprint]. 2024 Jul 25:2024.07.25.605170. doi: 10.1101/2024.07.25.605170. bioRxiv. 2024. PMID: 39372771 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

