Zinc oxide nanoparticles produced by Zingiber officinale ameliorates acute toxoplasmosis-induced pathological and biochemical alterations and reduced parasite burden in mice model
- PMID: 37410712
- PMCID: PMC10325114
- DOI: 10.1371/journal.pntd.0011447
Zinc oxide nanoparticles produced by Zingiber officinale ameliorates acute toxoplasmosis-induced pathological and biochemical alterations and reduced parasite burden in mice model
Abstract
Background: Although, approximately 30% of the world's population is estimated to be infected with Toxoplasma gondii (T. gondii) with serious manifestations in immunocompromised patients and pregnant females, the available treatment options for toxoplasmosis are limited with serious side effects. Therefore, it is of great importance to identify novel potent, well tolerated candidates for treatment of toxoplasmosis. The present study aimed to evaluate the effect of Zinc oxide nanoparticles (ZnO NPs) synthesized using Zingiber officinale against acute toxoplasmosis in experimentally infected mice.
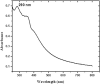
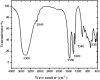
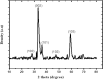
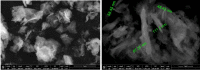
Methods: The ethanolic extract of ginger was used to prepare ZnO NPs. The produced ZnO NPs were characterized in terms of structure and morphology using Fourier Transformed Infrared Spectroscopy (FTIR), X-Ray Diffraction (XRD), UV- spectroscopy and scanning electron microscopy (SEM). The prepared formula was used in treatment of T. gondii RH virulent strain. Forty animals were divided into four groups, with ten mice per group. The first group was the uninfected, control group. The second group was infected but untreated. The third and the fourth groups received ZnO NPs and Spiramycin orally in a dose of 10 mg/kg and 200 mg/kg/day respectively. The effect of the used formulas on the animals survival rate, parasite burden, liver enzymes -including Alanine transaminase (ALT) and aspartate transaminase (AST)-, nitric oxide (NO) and Catalase antioxidant enzyme (CAT) activity was measured. Moreover, the effect of treatment on histopathological alterations associated with toxoplasmosis was examined.
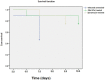
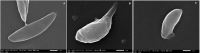
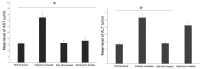
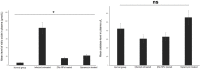
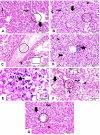
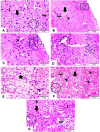
Results: Mice treated with ZnO NPs showed the longest survival time with significant reduction in the parasite load in the livers and peritoneal fluids of the same group. Moreover, ZnO NPs treatment was associated with a significant reduction in the level of liver enzymes (ALT, AST) and NO and a significant increase in the antioxidant activity of CAT enzyme. SEM examination of tachyzoites from the peritoneal fluid showed marked distortion of T. gondii tachyzoites isolated from mice treated with ZnO NPs in comparison to untreated group. T. gondii induced histopathological alterations in the liver and brain were reversed by ZnO NPs treatment with restoration of normal tissue morphology.
Conclusion: The produced formula showed a good therapeutic potential in treatment of murine toxoplasmosis as demonstrated by prolonged survival rate, reduced parasite burden, improved T. gondii associated liver injury and histopathological alterations. Thus, we assume that the protective effect observed in the current research is attributed to the antioxidant capability of NPs. Based on the results obtained from the current work, we suggest greenly produced ZnO NPs as a chemotherapeutic agent with good therapeutic potential and high levels of safety in the treatment of toxoplasmosis.
Copyright: © 2023 El-kady et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
The Therapeutic Efficacy of Zinc Oxide Nanoparticles on Acute Toxoplasmosis in BALB/c Mice.Iran J Parasitol. 2023 Oct-Dec;18(4):505-513. doi: 10.18502/ijpa.v18i4.14259. Iran J Parasitol. 2023. PMID: 38169550 Free PMC article.
-
Zingiber officinale Ameliorates Acute Toxoplasmosis-Induced Pathology in Mice.Acta Parasitol. 2024 Dec;69(4):1785-1800. doi: 10.1007/s11686-024-00884-1. Epub 2024 Sep 3. Acta Parasitol. 2024. PMID: 39225734
-
The powerful synergistic effect of spiramycin/propolis loaded chitosan/alginate nanoparticles on acute murine toxoplasmosis.PLoS Negl Trop Dis. 2022 Mar 16;16(3):e0010268. doi: 10.1371/journal.pntd.0010268. eCollection 2022 Mar. PLoS Negl Trop Dis. 2022. PMID: 35294434 Free PMC article.
-
Lipid metabolism: the potential targets for toxoplasmosis treatment.Parasit Vectors. 2024 Mar 6;17(1):111. doi: 10.1186/s13071-024-06213-9. Parasit Vectors. 2024. PMID: 38448975 Free PMC article. Review.
-
Toxoplasma gondii infection: novel emerging therapeutic targets.Expert Opin Ther Targets. 2023 Apr-May;27(4-5):293-304. doi: 10.1080/14728222.2023.2217353. Epub 2023 May 24. Expert Opin Ther Targets. 2023. PMID: 37212443 Free PMC article. Review.
Cited by
-
Comparative evaluation of live attenuated and killed tachyzoites as vaccine candidates for toxoplasmosis.AMB Express. 2025 Jul 10;15(1):102. doi: 10.1186/s13568-025-01889-3. AMB Express. 2025. PMID: 40637955 Free PMC article.
-
Phytogenic-Mediated Zinc Oxide Nanoparticles Using the Seed Extract of Citrullus lanatus and Its Integrated Potency against Multidrug Resistant Bacteria.ACS Omega. 2024 Mar 28;9(14):16832-16841. doi: 10.1021/acsomega.4c01554. eCollection 2024 Apr 9. ACS Omega. 2024. PMID: 38617622 Free PMC article.
-
Green synthesized Zingiber officinale-ZnO nanoparticles: anticancer efficacy against 3D breast cancer model.Future Sci OA. 2024 Dec 31;10(1):2419806. doi: 10.1080/20565623.2024.2419806. Epub 2024 Nov 14. Future Sci OA. 2024. PMID: 39539163 Free PMC article.
-
The Therapeutic Efficacy of Zinc Oxide Nanoparticles on Acute Toxoplasmosis in BALB/c Mice.Iran J Parasitol. 2023 Oct-Dec;18(4):505-513. doi: 10.18502/ijpa.v18i4.14259. Iran J Parasitol. 2023. PMID: 38169550 Free PMC article.
-
Zinc Oxide Nanoparticles as Next-Generation Feed Additives: Bridging Antimicrobial Efficacy, Growth Promotion, and Sustainable Strategies in Animal Nutrition.Nanomaterials (Basel). 2025 Jul 2;15(13):1030. doi: 10.3390/nano15131030. Nanomaterials (Basel). 2025. PMID: 40648737 Free PMC article. Review.
References
-
- Robert-Gangneux F, Sterkers Y, Yera H, Accoceberry I, Menotti J, Cassaing S, et al.. Molecular diagnosis of toxoplasmosis in immunocompromised patients: a 3-year multicenter retrospective study. J Clin Microbiol. 2015;53(5):1677–1684. Epub 2015/03/13. doi: 10.1128/JCM.03282-14 ; PubMed Central PMCID: PMC4400795. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

