Design of Tetrazolium Cations for the Release of Antiproliferative Formazan Chelators in Mammalian Cells
- PMID: 37410992
- PMCID: PMC10521327
- DOI: 10.1021/jacs.3c02033
Design of Tetrazolium Cations for the Release of Antiproliferative Formazan Chelators in Mammalian Cells
Abstract
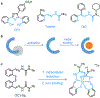
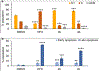
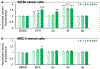
Cancer cells generally present a higher demand for iron, which plays crucial roles in tumor progression and metastasis. This iron addiction provides opportunities to develop broad spectrum anticancer drugs that target iron metabolism. In this context, prochelation approaches are investigated to release metal-binding compounds under specific conditions, thereby limiting off-target toxicity. Here, we demonstrate a prochelation strategy inspired by the bioreduction of tetrazolium cations widely employed to assess the viability of mammalian cells. We designed a series of tetrazolium-based compounds for the intracellular release of metal-binding formazan ligands. The combination of reduction potentials appropriate for intracellular reduction and an N-pyridyl donor on the formazan scaffold led to two effective prochelators. The reduced formazans bind as tridentate ligands and stabilize low-spin Fe(II) centers in complexes of 2:1 ligand-to-metal stoichiometry. The tetrazolium salts are stable in blood serum for over 24 h, and antiproliferative activities at micromolar levels were recorded in a panel of cancer cell lines. Additional assays confirmed the intracellular activation of the prochelators and their ability to affect cell cycle progression, induce apoptotic death, and interfere with iron availability. Demonstrating the role of iron in their intracellular effects, the prochelators impacted the expression levels of key iron regulators (i.e., transferrin receptor 1 and ferritin), and iron supplementation mitigated their cytotoxicity. Overall, this work introduces the tetrazolium core as a platform to build prochelators that can be tuned for activation in the reducing environment of cancer cells and produce antiproliferative formazan chelators that interfere with cellular iron homeostasis.
Figures


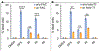
Similar articles
-
Quinoline-based tetrazolium prochelators: formazan release, iron sequestration, and antiproliferative efficacy in cancer cells.Chem Commun (Camb). 2024 Jun 11;60(48):6150-6153. doi: 10.1039/d4cc01523a. Chem Commun (Camb). 2024. PMID: 38804255 Free PMC article.
-
Thiol-Reactive Arylsulfonate Masks for Phenolate Donors in Antiproliferative Iron Prochelators.Inorg Chem. 2022 Dec 12;61(49):19974-19982. doi: 10.1021/acs.inorgchem.2c03250. Epub 2022 Dec 1. Inorg Chem. 2022. PMID: 36455205 Free PMC article.
-
Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives.Acta Histochem. 2018 Apr;120(3):159-167. doi: 10.1016/j.acthis.2018.02.005. Epub 2018 Feb 26. Acta Histochem. 2018. PMID: 29496266 Review.
-
Heterocyclic dithiocarbazate iron chelators: Fe coordination chemistry and biological activity.Dalton Trans. 2012 Jun 7;41(21):6536-48. doi: 10.1039/c2dt12387h. Epub 2012 Feb 24. Dalton Trans. 2012. PMID: 22362375
-
Tetrazolium salts and formazans.Prog Histochem Cytochem. 1976;9(3):1-56. doi: 10.1016/s0079-6336(76)80015-0. Prog Histochem Cytochem. 1976. PMID: 792958 Review.
Cited by
-
Disulfide-based 2-pyridyl-hydrazone prochelators induce iron deprivation and oxidative stress in ovarian cancer cells.J Biol Inorg Chem. 2025 Aug;30(4-5):443-452. doi: 10.1007/s00775-025-02119-8. Epub 2025 Jul 1. J Biol Inorg Chem. 2025. PMID: 40591007
-
Quinoline-based tetrazolium prochelators: formazan release, iron sequestration, and antiproliferative efficacy in cancer cells.Chem Commun (Camb). 2024 Jun 11;60(48):6150-6153. doi: 10.1039/d4cc01523a. Chem Commun (Camb). 2024. PMID: 38804255 Free PMC article.
References
-
- Richardson DR; Kalinowski DS; Lau S; Jansson PJ; Lovejoy DB Cancer cell iron metabolism and the development of potent iron chelators as anti-tumour agents. Biochim. Biophys. Acta 2009, 1790, 702–717. - PubMed
-
- Recalcati S; Gammella E; Cairo G Dysregulation of iron metabolism in cancer stem cells. Free Radic. Biol. Med 2019, 133, 216–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

