Sugar distributions on gangliosides guide the formation and stability of amyloid-β oligomers
- PMID: 37413816
- PMCID: PMC10529042
- DOI: 10.1016/j.bpc.2023.107073
Sugar distributions on gangliosides guide the formation and stability of amyloid-β oligomers
Abstract
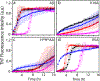
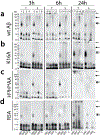
Aggregation of Aβ peptides is a key contributor to the etiology of Alzheimer's disease. Being intrinsically disordered, monomeric Aβ is susceptible to conformational excursions, especially in the presence of important interacting partners such as membrane lipids, to adopt specific aggregation pathways. Furthermore, components such as gangliosides in membranes and lipid rafts are known to play important roles in the adoption of pathways and the generation of discrete neurotoxic oligomers. Yet, what roles do carbohydrates on gangliosides play in this process remains unknown. Here, using GM1, GM3, and GD3 ganglioside micelles as models, we show that the sugar distributions and cationic amino acids within Aβ N-terminal region modulate oligomerization of Aβ temporally, and dictate the stability and maturation of oligomers. These results demonstrate the selectivity of sugar distributions on the membrane surface toward oligomerization of Aβ and thus implicate cell-selective enrichment of oligomers.
Keywords: Aggregation; Alzheimer's disease; Amyloid-β; Gangliosides; Oligomers.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no known competing financial interests or personal relationships that could have appeared to make an impact on the work reported in this paper.
Figures




Update of
-
Sugar distributions on gangliosides guide the formation and stability of amyloid-β oligomers.bioRxiv [Preprint]. 2023 May 10:2023.05.09.540003. doi: 10.1101/2023.05.09.540003. bioRxiv. 2023. Update in: Biophys Chem. 2023 Sep;300:107073. doi: 10.1016/j.bpc.2023.107073. PMID: 37214891 Free PMC article. Updated. Preprint.
Similar articles
-
Sugar distributions on gangliosides guide the formation and stability of amyloid-β oligomers.bioRxiv [Preprint]. 2023 May 10:2023.05.09.540003. doi: 10.1101/2023.05.09.540003. bioRxiv. 2023. Update in: Biophys Chem. 2023 Sep;300:107073. doi: 10.1016/j.bpc.2023.107073. PMID: 37214891 Free PMC article. Updated. Preprint.
-
Alzheimer's disease: NMR studies of asialo (GM1) and trisialo (GT1b) ganglioside interactions with Abeta(1-40) peptide in a membrane mimic environment.Neurochem Res. 2004 Feb;29(2):447-53. doi: 10.1023/b:nere.0000013750.80925.25. Neurochem Res. 2004. PMID: 15002743
-
Ganglioside Micelles Affect Amyloid β Aggregation by Coassembly.ACS Chem Neurosci. 2023 Dec 20;14(24):4335-4343. doi: 10.1021/acschemneuro.3c00524. Epub 2023 Dec 5. ACS Chem Neurosci. 2023. PMID: 38050745 Free PMC article.
-
Physicochemical interactions of amyloid beta-peptide with lipid bilayers.Biochim Biophys Acta. 2007 Aug;1768(8):1935-42. doi: 10.1016/j.bbamem.2007.02.009. Epub 2007 Feb 20. Biochim Biophys Acta. 2007. PMID: 17382287 Review.
-
[Ganglioside cluster-mediated aggregation and cytotoxicity of amyloid beta-peptide: molecular mechanism and inhibition].Yakugaku Zasshi. 2010 Apr;130(4):511-5. doi: 10.1248/yakushi.130.511. Yakugaku Zasshi. 2010. PMID: 20371994 Review. Japanese.
Cited by
-
De Novo Amyloid Peptide-Polymer Blends with Enhanced Mechanical and Biological Properties.ACS Appl Polym Mater. 2025 Mar 12;7(6):3739-3751. doi: 10.1021/acsapm.4c04020. eCollection 2025 Mar 28. ACS Appl Polym Mater. 2025. PMID: 40177395 Free PMC article.
-
Differential effects of ganglioside lipids on the conformation and aggregation of islet amyloid polypeptide.Protein Sci. 2024 Aug;33(8):e5119. doi: 10.1002/pro.5119. Protein Sci. 2024. PMID: 39012029 Free PMC article.
-
LL-37: Structures, Antimicrobial Activity, and Influence on Amyloid-Related Diseases.Biomolecules. 2024 Mar 8;14(3):320. doi: 10.3390/biom14030320. Biomolecules. 2024. PMID: 38540740 Free PMC article. Review.
-
The Influence of Lipid Electric Charge on the Binding of Aβ(1-42) Amyloid Peptide to Bilayers in the Liquid-Ordered State.Biomolecules. 2024 Mar 1;14(3):298. doi: 10.3390/biom14030298. Biomolecules. 2024. PMID: 38540718 Free PMC article.
-
Anionic lipid catalyzes the generation of cytotoxic insulin oligomers.bioRxiv [Preprint]. 2025 Jan 18:2025.01.14.633028. doi: 10.1101/2025.01.14.633028. bioRxiv. 2025. Update in: Biomolecules. 2025 Jul 11;15(7):994. doi: 10.3390/biom15070994. PMID: 39868250 Free PMC article. Updated. Preprint.
References
-
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kövari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG, Correlation of Alzheimer Disease Neuropathologic Changes With Cognitive Status: A Review of the Literature, J Neuropathol Exp Neurol. 71 (2012) 362–381. 10.1097/NEN.0b013e31825018f7. - DOI - PMC - PubMed
-
- Selkoe DJ, Alzheimer’s disease: genes, proteins, and therapy, Physiol Rev. 81 (2001) 741–766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

