Human embryo live imaging reveals nuclear DNA shedding during blastocyst expansion and biopsy
- PMID: 37413989
- PMCID: PMC11170958
- DOI: 10.1016/j.cell.2023.06.003
Human embryo live imaging reveals nuclear DNA shedding during blastocyst expansion and biopsy
Abstract
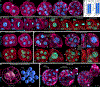
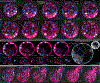
Proper preimplantation development is essential to assemble a blastocyst capable of implantation. Live imaging has uncovered major events driving early development in mouse embryos; yet, studies in humans have been limited by restrictions on genetic manipulation and lack of imaging approaches. We have overcome this barrier by combining fluorescent dyes with live imaging to reveal the dynamics of chromosome segregation, compaction, polarization, blastocyst formation, and hatching in the human embryo. We also show that blastocyst expansion mechanically constrains trophectoderm cells, causing nuclear budding and DNA shedding into the cytoplasm. Furthermore, cells with lower perinuclear keratin levels are more prone to undergo DNA loss. Moreover, applying trophectoderm biopsy, a mechanical procedure performed clinically for genetic testing, increases DNA shedding. Thus, our work reveals distinct processes underlying human development compared with mouse and suggests that aneuploidies in human embryos may not only originate from chromosome segregation errors during mitosis but also from nuclear DNA shedding.
Keywords: aneuploidy; human embryo; live imaging; preimplantation; trophectoderm biopsy.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.S. is on the Scientific Advisory Board of Igenomix, consults to ALIFE and Legacy and is Deputy Editor of Human Reproduction. C.S. is Scientific Advisor of Igenomix.
Figures




Comment in
-
Dyes illuminate live human embryogenesis.Cell. 2023 Jul 20;186(15):3143-3145. doi: 10.1016/j.cell.2023.06.011. Cell. 2023. PMID: 37478818
-
A peek into early human embryogenesis.Nat Methods. 2023 Aug;20(8):1130. doi: 10.1038/s41592-023-01982-0. Nat Methods. 2023. PMID: 37553402 No abstract available.
References
-
- Adamson GD, de Mouzon J, Chambers GM, Zegers-Hochschild F, Mansour R, Ishihara O, Banker M, and Dyer S (2018). International Committee for Monitoring Assisted Reproductive Technology: world report on assisted reproductive technology, 2011. Fertil. Steril. 110, 1067–1080. 10.1016/j.fertnstert.2018.06.039. - DOI - PubMed
-
- Fierro-Gonzalez JC, White MR, Silva J, and Plachta N (2013). Cadherin-dependent filopodia control preimplantation embryo compaction Nat. Cell Biol. 15, 1424–1433. - PubMed
-
- Samarage CR, White MD, Alvarez YD, Fierro-Gonzalez JC, Henon Y, Jesudason EC, Bissiere S, Fouras A, and Plachta N (2015). Cortical Tension Allocates the First Inner Cells of the Mammalian Embryo. Dev. Cell 34, 435–447. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

