Which patients with metastatic hormone-sensitive prostate cancer benefit from docetaxel: a systematic review and meta-analysis of individual participant data from randomised trials
- PMID: 37414011
- PMCID: PMC7616350
- DOI: 10.1016/S1470-2045(23)00230-9
Which patients with metastatic hormone-sensitive prostate cancer benefit from docetaxel: a systematic review and meta-analysis of individual participant data from randomised trials
Abstract
Background: Adding docetaxel to androgen deprivation therapy (ADT) improves survival in patients with metastatic, hormone-sensitive prostate cancer, but uncertainty remains about who benefits most. We therefore aimed to obtain up-to-date estimates of the overall effects of docetaxel and to assess whether these effects varied according to prespecified characteristics of the patients or their tumours.
Methods: The STOPCAP M1 collaboration conducted a systematic review and meta-analysis of individual participant data. We searched MEDLINE (from database inception to March 31, 2022), Embase (from database inception to March 31, 2022), the Cochrane Central Register of Controlled Trials (from database inception to March 31, 2022), proceedings of relevant conferences (from Jan 1, 1990, to Dec 31, 2022), and ClinicalTrials.gov (from database inception to March 28, 2023) to identify eligible randomised trials that assessed docetaxel plus ADT compared with ADT alone in patients with metastatic, hormone-sensitive prostate cancer. Detailed and updated individual participant data were requested directly from study investigators or through relevant repositories. The primary outcome was overall survival. Secondary outcomes were progression-free survival and failure-free survival. Overall pooled effects were estimated using an adjusted, intention-to-treat, two-stage, fixed-effect meta-analysis, with one-stage and random-effects sensitivity analyses. Missing covariate values were imputed. Differences in effect by participant characteristics were estimated using adjusted two-stage, fixed-effect meta-analysis of within-trial interactions on the basis of progression-free survival to maximise power. Identified effect modifiers were also assessed on the basis of overall survival. To explore multiple subgroup interactions and derive subgroup-specific absolute treatment effects we used one-stage flexible parametric modelling and regression standardisation. We assessed the risk of bias using the Cochrane Risk of Bias 2 tool. This study is registered with PROSPERO, CRD42019140591.
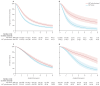
Findings: We obtained individual participant data from 2261 patients (98% of those randomised) from three eligible trials (GETUG-AFU15, CHAARTED, and STAMPEDE trials), with a median follow-up of 72 months (IQR 55-85). Individual participant data were not obtained from two additional small trials. Based on all included trials and patients, there were clear benefits of docetaxel on overall survival (hazard ratio [HR] 0·79, 95% CI 0·70 to 0·88; p<0·0001), progression-free survival (0·70, 0·63 to 0·77; p<0·0001), and failure-free survival (0·64, 0·58 to 0·71; p<0·0001), representing 5-year absolute improvements of around 9-11%. The overall risk of bias was assessed to be low, and there was no strong evidence of differences in effect between trials for all three main outcomes. The relative effect of docetaxel on progression-free survival appeared to be greater with increasing clinical T stage (pinteraction=0·0019), higher volume of metastases (pinteraction=0·020), and, to a lesser extent, synchronous diagnosis of metastatic disease (pinteraction=0·077). Taking into account the other interactions, the effect of docetaxel was independently modified by volume and clinical T stage, but not timing. There was no strong evidence that docetaxel improved absolute effects at 5 years for patients with low-volume, metachronous disease (-1%, 95% CI -15 to 12, for progression-free survival; 0%, -10 to 12, for overall survival). The largest absolute improvement at 5 years was observed for those with high-volume, clinical T stage 4 disease (27%, 95% CI 17 to 37, for progression-free survival; 35%, 24 to 47, for overall survival).
Interpretation: The addition of docetaxel to hormone therapy is best suited to patients with poorer prognosis for metastatic, hormone-sensitive prostate cancer based on a high volume of disease and potentially the bulkiness of the primary tumour. There is no evidence of meaningful benefit for patients with metachronous, low-volume disease who should therefore be managed differently. These results will better characterise patients most and, importantly, least likely to gain benefit from docetaxel, potentially changing international practice, guiding clinical decision making, better informing treatment policy, and improving patient outcomes.
Funding: UK Medical Research Council and Prostate Cancer UK.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests BT declares consulting fees from Amgen, Astellas, Bayer, Ferring, Novartis, and Janssen; payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from Amgen, Astellas, Bayer, Ferring, Novartis, Janssen, and Myovant during the past 36 months. CJS declares grants or contracts from Bayer, Sanofi, Astellas/Pfizer, Dendreon, and Janssen, and personal consulting fees from Bayer, Astellas, Janssen, CellCentric, PointBiopharma, Pfizer, Novartis, Genentech/Roche, Bristol Myers Squibb (BMS), Lilly, Henguiu, and AstraZeneca during the past 36 months. GG declares institutional grants from BMS; institutional payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from Janssen, Amgen, BMS, IPSEN, AAA, Curium, AstraZeneca, Bayer, Pfizer/Merck, and Astellas; personal payment for expert testimony from BMS, Bayer, and Pfizer/Merck; and institutional support for attending meetings or travel from Janssen, BMS, AstraZeneca, Bayer, and Pfizer/Merck during the past 36 months. GL declares payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from Sanofi-Genzyme; participation on a data safety monitoring board or advisory board from Janssen; and leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid, from ECOG-ACRIN (Prostate Cancer Subcommittee Chair) during the past 36 months. MRS declares grants or contracts from Astellas, Clovis Oncology, Janssen, Pfizer, Novartis, and Sanofi-Aventis (all on research that is independent of this manuscript); consulting fees from Eli Lilly; and payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from Lilly Oncology and Janssen (speaker fees not relating to any particular medicinal product) during the past 36 months. KF declares institutional honoraria for participation on advisory boards and in talks for Amgen, Astellas, AstraZeneca, Bayer, Clovis, Daiichi Sankyo, Janssen, MSD, Novartis/AAA, Pfizer, and Sanofi, and personal honoraria for participation in advisory boards with Arvinas, CureVac and Orion during the past 36 months. All other authors declare no competing interests.
Figures



References
-
- Gravis G, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:149–58. - PubMed
-
- Gravis G, Boher JM, Joly F, et al. Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur Urol. 2016;70:256–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

