Does maternal vitamin D status influence placental weight or vascular and inflammatory pathology? Secondary analysis from the Kellogg Pregnancy Study
- PMID: 37414103
- PMCID: PMC11229515
- DOI: 10.1016/j.jsbmb.2023.106358
Does maternal vitamin D status influence placental weight or vascular and inflammatory pathology? Secondary analysis from the Kellogg Pregnancy Study
Abstract
Introduction: Positive effects of vitamin D (vitD) supplementation on comorbidities of pregnancy (COP) have been explored; however, few studies have elucidated the pathophysiology behind the development of these COP and the potential relationship with derangements in placental development and morphology. Additionally, it is known that placentas weighing 10th-90th % for gestational age are associated with better outcomes. Therefore, the objective of this study was to assess the impact of resulting circulating serum 25(OH)D concentrations associated with intake of high or low doses of supplementary vitD on placental development and morphology in women who participated in a randomized double blind, placebo-controlled trial of vitD supplementation. We hypothesized that if maternal serum 25(OH)D concentration (vitD status marker) is insufficient/deficient, then placental weight and % for gestational age (GA) will be smaller and will correlate with increased vascular and inflammatory placental pathologic findings.
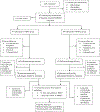
Methods: The findings of the present study are a secondary analysis of data generated from a previously reported randomized controlled trial (RCT), the Kellogg Vitamin D Pregnancy Study. Pregnant women (n = 297) in this RCT (January 2013 - April 2018) were randomly assigned to 400 IU vs. 4400 IU vitD/day (10-14 weeks' gestational age) and followed to delivery. 132 placentas were analyzed by pathologists blinded to treatment, and the 2016 Amsterdam Consensus Criteria were used to categorize grouping/grading of placental pathology and weight. Total [25(OH)D] was measured using radioimmunoassay (ng/mL). Chi-square and Student's t-test were used to show the difference in maternal characteristics by treatment group and by placental weight. Chi-square analysis was used to determine differences between the percent pathology findings by treatment group. Students t-test was used to determine the differences in vitD status and the frequency of placental lesions. Association between [25(OH)D] area under the curve (AUC) and placental morphology were determined in a regression model that included maternal BMI ≥ 30 kg/m2, race/ethnicity, and vitD treatment group allocation. Data were analyzed using SAS v9.4 (Cary, NC) and statistical significance was indicated by p < 0.05.
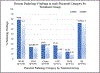
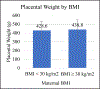
Results: The percent pathology findings by treatment group were not significantly different for each of the placental pathology categories as defined by the 2016 Amsterdam Consensus Criteria including placental weight. However, when using 25(OH)D as a biomarker for vitD status, linear regression model showed maternal serum [25(OH)D] AUC was significantly associated with greater placental weight (p = 0.023). Logistic regression models showed mothers with BMI ≥ 30 kg/m2 had larger placental weight (p = 0.046), and Hispanic and white/Caucasian mothers had greater placental weights than Black American mothers (p = 0.025). When placentas ≥ 90th % for GA, n = 7, were removed from the placental pool, Pearson correlation still showed a positive association between maternal serum 25(OH)D AUC and placental weight (p = 0.011). In a second linear regression model of placentas ≥ 90th % for GA (n = 7) vs. placentas < 90th % (n = 108), maternal serum 25(OH)D AUC was significantly greater in those placentas ≥ 90th % (p = 0.03); however, this was not associated with increased perinatal mortality. CONCLUSION FINDINGS: suggest increasing maternal serum [25(OH)D] via vitamin D supplementation during pregnancy did not adversely affect placental morphology; trends showed those in the treatment group had fewer placental lesions. Placental weight was found to be significantly associated with [25(OH)D] AUC, which represents maternal vitamin D status over the course of pregnancy; 7 placentas ≥ 90th % for GA were not associated with perinatal mortality.
Keywords: 25-hydroxyvitamin D; Inflammation; Placenta; Placental weight; Pregnancy; Vitamin D.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no conflict of interest.
Figures
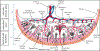


Similar articles
-
High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6-month follow-up pilot study.Breastfeed Med. 2006 Summer;1(2):59-70. doi: 10.1089/bfm.2006.1.59. Breastfeed Med. 2006. PMID: 17661565 Clinical Trial.
-
Vitamin D Supplementation Improves Mitochondrial Function and Reduces Inflammation in Placentae of Obese Women.Front Endocrinol (Lausanne). 2022 May 31;13:893848. doi: 10.3389/fendo.2022.893848. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35712242 Free PMC article.
-
Post Hoc Analysis of National Institute of Child Health and Human Development Vitamin-D Pregnancy Cohort and The Role of Functional Vitamin-D Deficiency in Pregnancy.Am J Perinatol. 2024 May;41(S 01):e2098-e2105. doi: 10.1055/a-2097-2098. Epub 2023 May 22. Am J Perinatol. 2024. PMID: 37216969
-
Maternal risk factors and newborn infant vitamin D status: a scoping literature review.Nutr Res. 2019 Mar;63:1-20. doi: 10.1016/j.nutres.2018.11.011. Epub 2018 Dec 7. Nutr Res. 2019. PMID: 30824393
-
Vitamin D supplementation for term breastfed infants to prevent vitamin D deficiency and improve bone health.Cochrane Database Syst Rev. 2020 Dec 11;12(12):CD013046. doi: 10.1002/14651858.CD013046.pub2. Cochrane Database Syst Rev. 2020. PMID: 33305822 Free PMC article.
Cited by
-
Associations Between Prenatal Vitamin D and Placental Gene Expression.J Nutr. 2024 Dec;154(12):3603-3614. doi: 10.1016/j.tjnut.2024.10.019. Epub 2024 Oct 12. J Nutr. 2024. PMID: 39401684 Free PMC article.
-
A cohort study of serum 25-hydroxyvitamin D levels and the risk of hyperlipidaemia in adults.Front Nutr. 2025 Jan 24;11:1492621. doi: 10.3389/fnut.2024.1492621. eCollection 2024. Front Nutr. 2025. PMID: 39925969 Free PMC article.
-
Vitamin D and Child Neurodevelopment-A Post Hoc Analysis.Nutrients. 2023 Oct 3;15(19):4250. doi: 10.3390/nu15194250. Nutrients. 2023. PMID: 37836534 Free PMC article. Clinical Trial.
-
The role of vitamin D deficiency in placental dysfunction: A systematic review.Metabol Open. 2025 Jan 31;25:100350. doi: 10.1016/j.metop.2025.100350. eCollection 2025 Mar. Metabol Open. 2025. PMID: 40034802 Free PMC article.
-
Low levels of Vitamin D during pregnancy associated with gestational diabetes mellitus and low birth weight: results from the MAASTHI birth cohort.Front Nutr. 2024 Jun 3;11:1352617. doi: 10.3389/fnut.2024.1352617. eCollection 2024. Front Nutr. 2024. PMID: 38887504 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

