Long-term outcomes to neoadjuvant pembrolizumab based on pathological response for patients with resectable stage III/IV cutaneous melanoma
- PMID: 37414215
- PMCID: PMC11232562
- DOI: 10.1016/j.annonc.2023.06.006
Long-term outcomes to neoadjuvant pembrolizumab based on pathological response for patients with resectable stage III/IV cutaneous melanoma
Erratum in
-
Corrigendum to 'Long-term outcomes to neoadjuvant pembrolizumab based on pathological response for patients with resectable stage III/IV cutaneous melanoma': [Annals of Oncology 34 (2023) 806-812].Ann Oncol. 2024 Aug;35(8):756. doi: 10.1016/j.annonc.2024.03.002. Epub 2024 Apr 13. Ann Oncol. 2024. PMID: 38614876 Free PMC article. No abstract available.
Abstract
Background: While neoadjuvant immunotherapy for melanoma has shown promising results, the data have been limited by a relatively short follow-up time, with most studies reporting 2-year outcomes. The goal of this study was to determine long-term outcomes for stage III/IV melanoma patients treated with neoadjuvant and adjuvant programmed cell death receptor 1 (PD-1) inhibition.
Patients and methods: This is a follow-up study of a previously published phase Ib clinical trial of 30 patients with resectable stage III/IV cutaneous melanoma who received one dose of 200 mg IV neoadjuvant pembrolizumab 3 weeks before surgical resection, followed by 1 year of adjuvant pembrolizumab. The primary outcomes were 5-year overall survival (OS), 5-year recurrence-free survival (RFS), and recurrence patterns.
Results: We report updated results at 5 years of follow-up with a median follow-up of 61.9 months. No deaths occurred in patients with a major pathological response (MPR, <10% viable tumor) or complete pathological response (pCR, no viable tumor) (n = 8), compared to a 5-year OS of 72.8% for the remainder of the cohort (P = 0.12). Two of eight patients with a pCR or MPR had a recurrence. Of the patients with >10% viable tumor remaining, 8 of 22 patients (36%) had a recurrence. Additionally, the median time to recurrence was 3.9 years for patients with ≤10% viable tumor and 0.6 years for patients with >10% viable tumor (P = 0.044).
Conclusions: The 5-year results from this trial represent the longest follow-up of a single-agent neoadjuvant PD-1 trial to date. Response to neoadjuvant therapy continues to be an important prognosticator with regard to OS and RFS. Additionally, recurrences in patients with pCR occur later and are salvageable, with a 5-year OS of 100%. These results demonstrate the long-term efficacy of single-agent neoadjuvant/adjuvant PD-1 blockade in patients with a pCR and the importance of long-term follow-up for these patients.
Trial registration: Clinicaltrials.gov, NCT02434354.
Keywords: follow-up studies; immunotherapy; melanoma; neoadjuvant therapy; programmed cell death 1 receptor.
Copyright © 2023. Published by Elsevier Ltd.
Conflict of interest statement
Disclosure GCK serves on an advisory board of Merck. All remaining authors have declared no conflicts of interest.
Figures
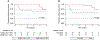
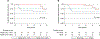
References
-
- Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med 2018;24(11):1655–1661. - PubMed
-
- Amaria RN, Prieto PA, Tetzlaff MT, et al. Neoadjuvant plus adjuvant dabrafenib and trametinib versus standard of care in patients with high-risk, surgically resectable melanoma: a single-centre, open-label, randomised, phase 2 trial. Lancet Oncol 2018;19(2):181–193. - PubMed
-
- Sharon CE, Karakousis GC. Educational review: neoadjuvant approaches to melanoma. Ann Surg Oncol 2022;29(13):8492–8500. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

