Differential roles of FOXC2 in the trabecular meshwork and Schlemm's canal in glaucomatous pathology
- PMID: 37414529
- PMCID: PMC10326420
- DOI: 10.26508/lsa.202201721
Differential roles of FOXC2 in the trabecular meshwork and Schlemm's canal in glaucomatous pathology
Abstract
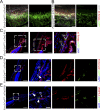
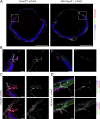
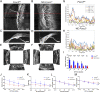
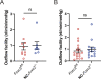
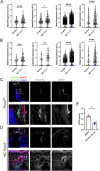
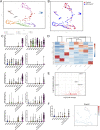
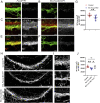
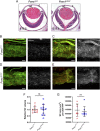
Impaired development and maintenance of Schlemm's canal (SC) are associated with perturbed aqueous humor outflow and intraocular pressure. The angiopoietin (ANGPT)/TIE2 signaling pathway regulates SC development and maintenance, whereas the molecular mechanisms of crosstalk between SC and the neural crest (NC)-derived neighboring tissue, the trabecular meshwork (TM), are poorly understood. Here, we show NC-specific forkhead box (Fox)c2 deletion in mice results in impaired SC morphogenesis, loss of SC identity, and elevated intraocular pressure. Visible-light optical coherence tomography analysis further demonstrated functional impairment of the SC in response to changes in intraocular pressure in NC-Foxc2 -/- mice, suggesting altered TM biomechanics. Single-cell RNA-sequencing analysis identified that this phenotype is predominately characterized by transcriptional changes associated with extracellular matrix organization and stiffness in TM cell clusters, including increased matrix metalloproteinase expression, which can cleave the TIE2 ectodomain to produce soluble TIE2. Moreover, endothelial-specific Foxc2 deletion impaired SC morphogenesis because of reduced TIE2 expression, which was rescued by deleting the TIE2 phosphatase VE-PTP. Thus, Foxc2 is critical in maintaining SC identity and morphogenesis via TM-SC crosstalk.
© 2023 Ujiie et al.
Conflict of interest statement
The authors declare the following competing interests: SE Quaggin is an inventor of patents related to therapeutic targeting of the ANGPT-TEK pathway in ocular hypertension and glaucoma and owns stock in and is a director of Mannin Research. SE Quaggin also receives consulting fees from AstraZeneca, Hanssen, the Lowry Medical Research Foundation, and Roche/Genentech; is Chair of the External Scientific Advisory Board for AstraZeneca; and is a scientific advisor or member of AstraZeneca, Genentech/Roche, the Karolinska CVRM Institute, the Lowry Medical Research Institute, Mannin, and Novartis. HF Zhang has financial interests in Opticent, Inc. The other authors declare no competing interests.
Figures









Similar articles
-
Transcriptomic profiling of Schlemm's canal cells reveals a lymphatic-biased identity and three major cell states.Elife. 2024 Oct 18;13:RP96459. doi: 10.7554/eLife.96459. Elife. 2024. PMID: 39422453 Free PMC article.
-
Low Intraocular Pressure Induces Fibrotic Changes in the Trabecular Meshwork and Schlemm's Canal of Sprague Dawley Rats.Transl Vis Sci Technol. 2024 Oct 1;13(10):10. doi: 10.1167/tvst.13.10.10. Transl Vis Sci Technol. 2024. PMID: 39374003 Free PMC article.
-
A Histomorphometric and Computational Investigation of the Stabilizing Role of Pectinate Ligaments in the Aqueous Outflow Pathway.J Biomech Eng. 2024 Aug 1;146(8):081011. doi: 10.1115/1.4065164. J Biomech Eng. 2024. PMID: 38529724
-
Biomechanics of Schlemm's canal endothelium and intraocular pressure reduction.Prog Retin Eye Res. 2015 Jan;44:86-98. doi: 10.1016/j.preteyeres.2014.08.002. Epub 2014 Sep 16. Prog Retin Eye Res. 2015. PMID: 25223880 Free PMC article. Review.
-
Schlemm's Canal Surgery in Juvenile-Onset Open-Angle Glaucoma: A Narrative Review.Ophthalmic Res. 2025 Jul 30:1-28. doi: 10.1159/000547682. Online ahead of print. Ophthalmic Res. 2025. PMID: 40738090 Review.
Cited by
-
FOXC1 regulates endothelial CD98 (LAT1/4F2hc) expression in retinal angiogenesis and blood-retina barrier formation.Nat Commun. 2024 May 16;15(1):4097. doi: 10.1038/s41467-024-48134-2. Nat Commun. 2024. PMID: 38755144 Free PMC article.
-
Schlemm's canal-selective Tie2/TEK knockdown induces sustained ocular hypertension in adult mice.Exp Eye Res. 2024 Nov;248:110114. doi: 10.1016/j.exer.2024.110114. Epub 2024 Oct 3. Exp Eye Res. 2024. PMID: 39368692
-
Transcriptomic profiling of Schlemm's canal cells reveals a lymphatic-biased identity and three major cell states.Elife. 2024 Oct 18;13:RP96459. doi: 10.7554/eLife.96459. Elife. 2024. PMID: 39422453 Free PMC article.
-
The Advance of Single-Cell RNA Sequencing Applications in Ocular Physiology and Disease Research.Biomolecules. 2025 Aug 4;15(8):1120. doi: 10.3390/biom15081120. Biomolecules. 2025. PMID: 40867565 Free PMC article. Review.
-
Transcriptomic profiling of Schlemm's canal cells reveals a lymphatic-biased identity and three major cell states.bioRxiv [Preprint]. 2024 Aug 6:2023.08.31.555823. doi: 10.1101/2023.08.31.555823. bioRxiv. 2024. Update in: Elife. 2024 Oct 18;13:RP96459. doi: 10.7554/eLife.96459. PMID: 37886472 Free PMC article. Updated. Preprint.
References
-
- Bourne R, Steinmetz JD, Flaxman S, Briant PS, Taylor HR, Resnikoff S, Casson RJ, Abdoli A, Abu-Gharbieh E, Afshin A, et al. (2021) Trends in prevalence of blindness and distance and near vision impairment over 30 years: An analysis for the global burden of disease study. Lancet Glob Health 9: e130–e143. 10.1016/s2214-109x(20)30425-3 - DOI - PMC - PubMed
-
- Steinmetz JD, Bourne RRA, Briant PS, Flaxman SR, Taylor HRB, Jonas JB, Abdoli AA, Abrha WA, Abualhasan A, Abu-Gharbieh EG, et al. (2021) Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The right to sight: An analysis for the global burden of disease study. Lancet Glob Health 9: e144–e160. 10.1016/s2214-109x(20)30489-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 EY005722/EY/NEI NIH HHS/United States
- R01 EY026078/EY/NEI NIH HHS/United States
- R01 HL144129/HL/NHLBI NIH HHS/United States
- R01 EY030124/EY/NEI NIH HHS/United States
- R01 EY022359/EY/NEI NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- R01 EY028304/EY/NEI NIH HHS/United States
- T32 HL094293/HL/NHLBI NIH HHS/United States
- R01 EY034740/EY/NEI NIH HHS/United States
- T32 GM142604/GM/NIGMS NIH HHS/United States
- R01 EY029121/EY/NEI NIH HHS/United States
- U01 EY033001/EY/NEI NIH HHS/United States
- R01 EY031710/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
