Distinct Frontoparietal Brain Dynamics Underlying the Co-Occurrence of Autism and ADHD
- PMID: 37414550
- PMCID: PMC10348445
- DOI: 10.1523/ENEURO.0146-23.2023
Distinct Frontoparietal Brain Dynamics Underlying the Co-Occurrence of Autism and ADHD
Abstract
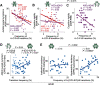
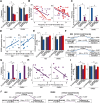
Previous diagnostic systems precluded the co-existence of autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) in one person; but, after many clinical reports, the diagnostic criteria were updated to allow their co-occurrence. Despite such a clinical change, the neurobiological bases underpinning the comorbidity remain poorly understood, and whether the ASD+ADHD condition is a simple overlap of the two disorders is unknown. Here, to answer this question, we compared the brain dynamics of high-functioning ASD+ADHD children with age-/sex-/IQ-matched pure ASD, pure ADHD, and typically developing (TD) children. Regarding autistic traits, the socio-communicational symptom of the ASD+ADHD children was explained by the same overstable brain dynamics as seen in pure ASD. In contrast, their ADHD-like traits were grounded on a unique neural mechanism that was unseen in pure ADHD: the core symptoms of pure ADHD were associated with the overly flexible whole-brain dynamics that were triggered by the unstable activity of the dorsal-attention network and the left parietal cortex; by contrast, the ADHD-like cognitive instability of the ASD+ADHD condition was correlated with the atypically frequent neural transition along a specific brain state pathway, which was induced by the atypically unstable activity of the frontoparietal control network and the left prefrontal cortex. These observations need to be validated in future studies using more direct and comprehensive behavioral indices, but the current findings suggest that the ASD+ADHD comorbidity is not a mere overlap of the two disorders. Particularly, its ADHD-like traits could represent a unique condition that would need a specific diagnosis and bespoke treatments.
Keywords: ADHD; MRI; autism; comorbidity; energy landscape analysis; intrinsic neural timescale.
Copyright © 2023 Watanabe and Watanabe.
Figures





Similar articles
-
Effects of the Co-occurrence of Anxiety and Attention-Deficit/Hyperactivity Disorder on Intrinsic Functional Network Centrality among Children with Autism Spectrum Disorder.Autism Res. 2019 Jul;12(7):1057-1068. doi: 10.1002/aur.2120. Epub 2019 May 10. Autism Res. 2019. PMID: 31074587
-
Decreased interoceptive accuracy in children with autism spectrum disorder and with comorbid attention deficit/hyperactivity disorder.Autism Res. 2022 Apr;15(4):729-739. doi: 10.1002/aur.2679. Epub 2022 Jan 27. Autism Res. 2022. PMID: 35088528
-
Functional brain network alterations in the co-occurrence of autism spectrum disorder and attention deficit hyperactivity disorder.Eur Child Adolesc Psychiatry. 2024 Feb;33(2):369-380. doi: 10.1007/s00787-023-02165-0. Epub 2023 Feb 17. Eur Child Adolesc Psychiatry. 2024. PMID: 36800038
-
[Autism spectrum disorder and attention-deficit/hyperactivity disorder: challenge in diagnosis and treatment].Medicina (B Aires). 2022 Aug 30;82 Suppl 3:67-70. Medicina (B Aires). 2022. PMID: 36054861 Review. Spanish.
-
[Comorbidity of autism spectrum disorder and attention deficit with hyperactivity. A review study].Rev Neurol. 2015 Feb 25;60 Suppl 1:S37-43. Rev Neurol. 2015. PMID: 25726822 Review. Spanish.
Cited by
-
Aberrant brain dynamics of large-scale functional networks across schizophrenia and mood disorder.Neuroimage Clin. 2024;41:103574. doi: 10.1016/j.nicl.2024.103574. Epub 2024 Feb 10. Neuroimage Clin. 2024. PMID: 38346380 Free PMC article.
-
Comparison of Large Language Model with Aphasia.Adv Sci (Weinh). 2025 Jun;12(22):e2414016. doi: 10.1002/advs.202414016. Epub 2025 May 14. Adv Sci (Weinh). 2025. PMID: 40369908 Free PMC article.
-
Noninvasive reduction of neural rigidity alters autistic behaviors in humans.Nat Neurosci. 2025 Jun;28(6):1348-1360. doi: 10.1038/s41593-025-01961-y. Epub 2025 Jun 6. Nat Neurosci. 2025. PMID: 40481227 Free PMC article.
-
Deep-Learning-Based Analysis Reveals a Social Behavior Deficit in Mice Exposed Prenatally to Nicotine.Cells. 2024 Feb 1;13(3):275. doi: 10.3390/cells13030275. Cells. 2024. PMID: 38334667 Free PMC article.
-
Clustering Analysis of Cognitive Profiles of Clinical Groups Using the CAS: An Examination of Japanese Clinical Populations.J Intell. 2025 Jun 19;13(6):71. doi: 10.3390/jintelligence13060071. J Intell. 2025. PMID: 40558821 Free PMC article.
References
-
- American Psychiatric Association (2022) Diagnostic and statistical manual of mental disorders, DSM-5-TR. Washington, DC: Amer Psychiatric Pub Inc.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
