Long-term Outcomes from a Phase 2 Study of Neoadjuvant Chemotherapy for Muscle-invasive Bladder Cancer (SWOG S1314; NCT02177695)
- PMID: 37414705
- PMCID: PMC10659139
- DOI: 10.1016/j.eururo.2023.06.014
Long-term Outcomes from a Phase 2 Study of Neoadjuvant Chemotherapy for Muscle-invasive Bladder Cancer (SWOG S1314; NCT02177695)
Abstract
Background: The COXEN gene expression model was evaluated for prediction of response to neoadjuvant chemotherapy for muscle-invasive bladder cancer (MIBC).
Objective: To conduct a secondary analysis of the association of each COXEN score with event-free survival (EFS) and overall survival (OS) and by treatment arm.
Design, setting, and participants: This was a randomized phase 2 trial of neoadjuvant gemcitabine-cisplatin (GC) or dose-dense methotrexate-vinblastine-adriamycin-cisplatin (ddMVAC) in MIBC.
Intervention: Patients were randomized to ddMVAC (every 14 d) or GC (every 21 d), both for four cycles.
Outcome measurements and statistical analysis: EFS events were defined as progression or death before scheduled surgery, a decision to not undergo surgery, recurrence, or death due to any cause after surgery. Cox regression was used to evaluate the COXEN score or treatment arm association with EFS and OS.
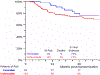
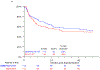
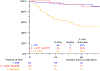
Results and limitations: A total of 167 evaluable patients were included in the COXEN analysis. The COXEN scores were not significantly prognostic for OS or EFS in the respective arms, but the GC COXEN score had a hazard ratio (HR) of 0.45 (95% confidence interval [CI] 0.20-0.99; p = 0.047) when the arms were pooled. In the intent-to-treat analysis (n = 227), there was no significant difference between ddMVAC and GC for OS (HR 0.87, 95% CI 0.54-1.40; p = 0.57) or EFS (HR 0.86, 95% CI 0.59-1.26; p = 0.45). Among the 192 patients who underwent surgery, pathologic response (pT0 vs downstaging vs no response) was strongly correlated with superior postsurgical survival (5-yr OS 90%, 89% and 52%, respectively).
Conclusions: The COXEN GC score has prognostic value for patients receiving cisplatin-based neoadjuvant treatment. The randomized, prospective design provides estimates of OS and EFS for GC and ddMVAC in this population. Pathologic response (<pT2) performed well as an intermediate endpoint in this modern cohort. For expediency in evaluating new regimens, pathologic response should continue to be used in phase 2 trials.
Patient summary: In this study, we evaluated a biomarker to predict the response to chemotherapy. The results did not meet the preset study parameters, but our study provides information on clinical outcomes with the use of chemotherapy before surgery for bladder cancer.
Trial registration: ClinicalTrials.gov NCT02177695.
Keywords: Biomarkers; Cystectomy; Neoadjuvant chemotherapy; Urinary bladder cancer.
Copyright © 2023 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures

Comment in
-
Biomarker challenges in the pursuit of personalized neoadjuvant chemotherapy for muscle-invasive bladder cancer: conclusions from SWOG S1314.Transl Androl Urol. 2024 Mar 31;13(3):458-462. doi: 10.21037/tau-23-620. Epub 2024 Mar 12. Transl Androl Urol. 2024. PMID: 38590957 Free PMC article. No abstract available.
References
-
- Flaig TW, Spiess PE, Agarwal N, et al. Bladder cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 2020;18:329–54. - PubMed
-
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859–66. - PubMed
-
- Zaid HB, Patel SG, Stimson CJ, et al. Trends in the utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer: results from the National Cancer Database. Urology 2014;83:75–80. - PubMed
-
- Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 2006;42:50–4. - PubMed
-
- von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000;18:3068–77. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

