A panel dataset of COVID-19 vaccination policies in 185 countries
- PMID: 37414885
- PMCID: PMC10444623
- DOI: 10.1038/s41562-023-01615-8
A panel dataset of COVID-19 vaccination policies in 185 countries
Abstract
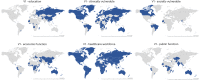
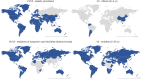
We present a panel dataset of COVID-19 vaccine policies, with data from 01 January 2020 for 185 countries and a number of subnational jurisdictions, reporting on vaccination prioritization plans, eligibility and availability, cost to the individual and mandatory vaccination policies. For each of these indicators, we recorded who is targeted by a policy using 52 standardized categories. These indicators document a detailed picture of the unprecedented scale of international COVID-19 vaccination rollout and strategy, indicating which countries prioritized and vaccinated which groups, when and in what order. We highlight key descriptive findings from these data to demonstrate uses for the data and to encourage researchers and policymakers in future research and vaccination planning. Numerous patterns and trends begin to emerge. For example: 'eliminator' countries (those that aimed to prevent virus entry into the country and community transmission) tended to prioritize border workers and economic sectors, while 'mitigator' countries (those that aimed to reduce the impact of community transmission) tended to prioritize the elderly and healthcare sectors for the first COVID-19 vaccinations; high-income countries published prioritization plans and began vaccinations earlier than low- and middle-income countries. Fifty-five countries were found to have implemented at least one policy of mandatory vaccination. We also demonstrate the value of combining this data with vaccination uptake rates, vaccine supply and demand data, and with further COVID-19 epidemiological data.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Tanne, J. H. Covid-19: Moderna will ask for authorisation for vaccine for infants and children. BMJ10.1136/bmj.o798 (2022). - PubMed
-
- Haas EJ, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

