SARS-CoV-2-specific immune responses and clinical outcomes after COVID-19 vaccination in patients with immune-suppressive disease
- PMID: 37414897
- PMCID: PMC10353927
- DOI: 10.1038/s41591-023-02414-4
SARS-CoV-2-specific immune responses and clinical outcomes after COVID-19 vaccination in patients with immune-suppressive disease
Abstract
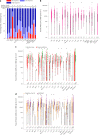
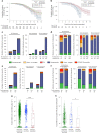
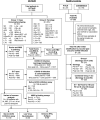
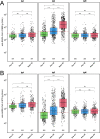
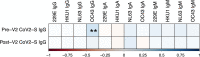
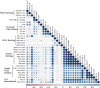
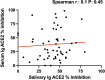
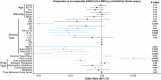
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immune responses and infection outcomes were evaluated in 2,686 patients with varying immune-suppressive disease states after administration of two Coronavirus Disease 2019 (COVID-19) vaccines. Overall, 255 of 2,204 (12%) patients failed to develop anti-spike antibodies, with an additional 600 of 2,204 (27%) patients generating low levels (<380 AU ml-1). Vaccine failure rates were highest in ANCA-associated vasculitis on rituximab (21/29, 72%), hemodialysis on immunosuppressive therapy (6/30, 20%) and solid organ transplant recipients (20/81, 25% and 141/458, 31%). SARS-CoV-2-specific T cell responses were detected in 513 of 580 (88%) patients, with lower T cell magnitude or proportion in hemodialysis, allogeneic hematopoietic stem cell transplantation and liver transplant recipients (versus healthy controls). Humoral responses against Omicron (BA.1) were reduced, although cross-reactive T cell responses were sustained in all participants for whom these data were available. BNT162b2 was associated with higher antibody but lower cellular responses compared to ChAdOx1 nCoV-19 vaccination. We report 474 SARS-CoV-2 infection episodes, including 48 individuals with hospitalization or death from COVID-19. Decreased magnitude of both the serological and the T cell response was associated with severe COVID-19. Overall, we identified clinical phenotypes that may benefit from targeted COVID-19 therapeutic strategies.
© 2023. The Author(s).
Conflict of interest statement
E.B. consults for AstaZeneca, Roche and Vaccitech and has patents in ChAdox1 HBV and HCV vaccines. I.B.M. receives consulting fees and honoraria from AbbVie, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Cabaletta, Causeway Therapeutics, Celgene, Evelo, Gilead, Janssen, Pfizer, Novartis, Eli Lilly, Moonlake and UCB Pharma and research support from Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Janssen, Novartis and UCB Pharma. C.S.G. has received research grants from AstraZeneca but unrelated to any of the work described in this manuscript. Furthermore, the funder had no specific role in the conceptualization, design, data collection, analysis, decision to publish or preparation of the manuscript. P. Klenerman was part of an advisory board for AstraZeneca for work unrelated to vaccines. L.L. acted as a paid advisor to Pfizer and AstraZeneca for work unrelated to vaccines and was on an advisory board for AstraZeneca for pre-exposure prophylaxis. L.L. is a Trustee of Kidney Research UK and is associated with the UK Kidney Association. The authors declare no other relevant conflicts of interest. The OCTAVE study group comprises investigators, sub-investigators and clinical trial staff. The PITCH consortium comprises investigators and sub-investigators in the UK Department of Health and Social Care-funded study.
Figures




References
-
- Office for National Statistics. People with long-term health conditions, UK: January to December 2019. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/... (2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

