ACEI/ARB and beta-blocker therapies for preventing cardiotoxicity of antineoplastic agents in breast cancer: a systematic review and meta-analysis
- PMID: 37414918
- PMCID: PMC10575808
- DOI: 10.1007/s10741-023-10328-z
ACEI/ARB and beta-blocker therapies for preventing cardiotoxicity of antineoplastic agents in breast cancer: a systematic review and meta-analysis
Abstract
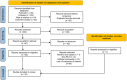
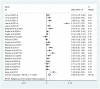
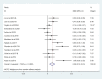
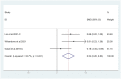
Anthracyclines and trastuzumab are widely used to treat breast cancer but increase the risk of cardiomyopathy and heart failure. With the use of trastuzumab and anthracycline-containing medications, this study intends to evaluate the effectiveness and security of current treatments against cardiotoxicity. We conducted a systematic review of randomized controlled trials (RCTs), which used at least one angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB), or beta-blocker (BB) to prevent cardiotoxicity of antineoplastic agents for breast cancer, in 4 databases (PubMed, Cochrane Library, EMBASE, Web of Science) from inception to 11 May 2022, without language restrictions. The outcome of interest was left ventricular ejection fraction (LVEF) and adverse events. Stata 15 and R software 4.2.1 were used to perform all statistical analyses. The Cochrane version 2 of the risk of bias tool was used to assess the risk of bias, and the grading of recommendations assessment, development, and evaluation (GRADE) assessment was used to appraise the quality of the evidence. Fifteen randomized clinical studies with a total of 1977 patients were included in the analysis. The included studies demonstrated statistically significant LVEF in the ACEI/ARB and BB treatment groups (χ2 = 184.75, I2 = 88.6%, p = 0.000; SMD 0.556, 95% CI 0.299 to 0.813). In an exploratory subgroup analysis, the benefit of experimental agents on LVEF, whether anthracyclines or trastuzumab, was prominent in patients treated with ACEIs, ARBs, and BBs. Compared to placebo, ACEI/ARB and BB treatments in breast cancer patients protect against cardiotoxicity after trastuzumab and anthracycline-containing medication treatment, indicating a benefit for both.
Keywords: ACEI/ARB; Beta-blocker; Breast cancer; Cardiotoxicity; LVEF.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Prevention of anthracycline and trastuzumab-induced decline in left ventricular ejection fraction with angiotensin-converting enzyme inhibitors or angiotensin receptor blocker: a narrative systematic review of randomised controlled trials.Intern Med J. 2024 Aug;54(8):1254-1263. doi: 10.1111/imj.16437. Epub 2024 Jun 14. Intern Med J. 2024. PMID: 38874281
-
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for adults with early (stage 1 to 3) non-diabetic chronic kidney disease.Cochrane Database Syst Rev. 2011 Oct 5;(10):CD007751. doi: 10.1002/14651858.CD007751.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2023 Jul 19;7:CD007751. doi: 10.1002/14651858.CD007751.pub3. PMID: 21975774 Updated.
-
Dexrazoxane for preventing or reducing cardiotoxicity in adults and children with cancer receiving anthracyclines.Cochrane Database Syst Rev. 2022 Sep 27;9(9):CD014638. doi: 10.1002/14651858.CD014638.pub2. Cochrane Database Syst Rev. 2022. PMID: 36162822 Free PMC article.
-
Beta-blockers and inhibitors of the renin-angiotensin aldosterone system for chronic heart failure with preserved ejection fraction.Cochrane Database Syst Rev. 2018 Jun 28;6(6):CD012721. doi: 10.1002/14651858.CD012721.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2021 May 22;5:CD012721. doi: 10.1002/14651858.CD012721.pub3. PMID: 29952095 Free PMC article. Updated.
-
Comparing Renin-Angiotensin-Aldosterone Blockade Regimens for Long-Term Chemotherapy-Related Cardiac Dysfunction: A Network Meta-Analysis.Cardiovasc Drugs Ther. 2025 Feb;39(1):171-186. doi: 10.1007/s10557-023-07457-w. Epub 2023 Jun 14. Cardiovasc Drugs Ther. 2025. PMID: 37314568
Cited by
-
Thinking Outside the Therapeutic Box: The Potential of Polyphenols in Preventing Chemotherapy-Induced Endothelial Dysfunction.Cells. 2025 Apr 9;14(8):566. doi: 10.3390/cells14080566. Cells. 2025. PMID: 40277892 Free PMC article. Review.
-
Natural language processing of electronic medical records identifies cardioprotective agents for anthracycline induced cardiotoxicity.Sci Rep. 2025 Feb 24;15(1):6678. doi: 10.1038/s41598-025-91187-6. Sci Rep. 2025. PMID: 39994365 Free PMC article.
-
Cardiotoxicity in Breast Cancer: Impact of Clinical Classifications and Treatment on Heart Health.Cancers (Basel). 2024 Dec 23;16(24):4281. doi: 10.3390/cancers16244281. Cancers (Basel). 2024. PMID: 39766180 Free PMC article.
-
Cancer-Therapy-Related Cardiac Dysfunction: Latest Advances in Prevention and Treatment.Life (Basel). 2025 Mar 15;15(3):471. doi: 10.3390/life15030471. Life (Basel). 2025. PMID: 40141815 Free PMC article. Review.
-
Association Between Advanced TNM Stages and Increased Risk of Cardiac Dysfunction in Patients with LVEF < 50.Medicina (Kaunas). 2025 Feb 10;61(2):301. doi: 10.3390/medicina61020301. Medicina (Kaunas). 2025. PMID: 40005419 Free PMC article.
References
-
- Valero V, Forbes J, Pegram MD, Pienkowski T, Eiermann W, von Minckwitz G, et al. Multicenter phase III randomized trial comparing docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab as first-line chemotherapy for patients with HER2-gene-amplified metastatic breast cancer (BCIRG 007 study): two highly active therapeutic regimens. J Clin Oncol. 2011;29(2):149–156. doi: 10.1200/JCO.2010.28.6450. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

