Removal of senescent cells reduces the viral load and attenuates pulmonary and systemic inflammation in SARS-CoV-2-infected, aged hamsters
- PMID: 37414987
- PMCID: PMC10353934
- DOI: 10.1038/s43587-023-00442-w
Removal of senescent cells reduces the viral load and attenuates pulmonary and systemic inflammation in SARS-CoV-2-infected, aged hamsters
Abstract
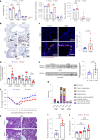
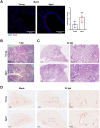
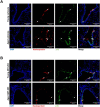
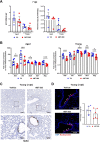
Older age is one of the strongest risk factors for severe COVID-19. In this study, we determined whether age-associated cellular senescence contributes to the severity of experimental COVID-19. Aged golden hamsters accumulate senescent cells in the lungs, and the senolytic drug ABT-263, a BCL-2 inhibitor, depletes these cells at baseline and during SARS-CoV-2 infection. Relative to young hamsters, aged hamsters had a greater viral load during the acute phase of infection and displayed higher levels of sequelae during the post-acute phase. Early treatment with ABT-263 lowered pulmonary viral load in aged (but not young) animals, an effect associated with lower expression of ACE2, the receptor for SARS-CoV-2. ABT-263 treatment also led to lower pulmonary and systemic levels of senescence-associated secretory phenotype factors and to amelioration of early and late lung disease. These data demonstrate the causative role of age-associated pre-existing senescent cells on COVID-19 severity and have clear clinical relevance.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






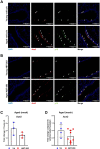
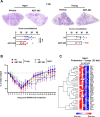
Comment in
-
Senolytics combat COVID-19 in aging.Nat Aging. 2023 Jul;3(7):762-763. doi: 10.1038/s43587-023-00450-w. Nat Aging. 2023. PMID: 37414986 Free PMC article.
References
-
- O’Driscoll M, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590:140–145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

