Advancements in clinical aspects of targeted therapy and immunotherapy in breast cancer
- PMID: 37415164
- PMCID: PMC10324146
- DOI: 10.1186/s12943-023-01805-y
Advancements in clinical aspects of targeted therapy and immunotherapy in breast cancer
Abstract
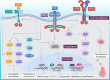
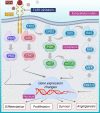
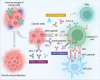
Breast cancer is the second leading cause of death for women worldwide. The heterogeneity of this disease presents a big challenge in its therapeutic management. However, recent advances in molecular biology and immunology enable to develop highly targeted therapies for many forms of breast cancer. The primary objective of targeted therapy is to inhibit a specific target/molecule that supports tumor progression. Ak strain transforming, cyclin-dependent kinases, poly (ADP-ribose) polymerase, and different growth factors have emerged as potential therapeutic targets for specific breast cancer subtypes. Many targeted drugs are currently undergoing clinical trials, and some have already received the FDA approval as monotherapy or in combination with other drugs for the treatment of different forms of breast cancer. However, the targeted drugs have yet to achieve therapeutic promise against triple-negative breast cancer (TNBC). In this aspect, immune therapy has come up as a promising therapeutic approach specifically for TNBC patients. Different immunotherapeutic modalities including immune-checkpoint blockade, vaccination, and adoptive cell transfer have been extensively studied in the clinical setting of breast cancer, especially in TNBC patients. The FDA has already approved some immune-checkpoint blockers in combination with chemotherapeutic drugs to treat TNBC and several trials are ongoing. This review provides an overview of clinical developments and recent advancements in targeted therapies and immunotherapies for breast cancer treatment. The successes, challenges, and prospects were critically discussed to portray their profound prospects.
Keywords: Breast cancer; Clinical trials; Immune-checkpoint Inhibitors; Immunotherapy; Targeted therapies.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

