Cancer-associated fibroblast-derived PAI-1 promotes lymphatic metastasis via the induction of EndoMT in lymphatic endothelial cells
- PMID: 37415190
- PMCID: PMC10324144
- DOI: 10.1186/s13046-023-02714-0
Cancer-associated fibroblast-derived PAI-1 promotes lymphatic metastasis via the induction of EndoMT in lymphatic endothelial cells
Abstract
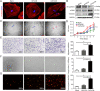
Background: Endothelial-mesenchymal transition (EndoMT) is an emerging adaptive process that modulates lymphatic endothelial function to drive aberrant lymphatic vascularization in the tumour microenvironment (TME); however, the molecular determinants that govern the functional role of EndoMT remain unclear. Here, we show that cancer-associated fibroblast (CAF)-derived PAI-1 promoted the EndoMT of lymphatic endothelial cells (LECs) in cervical squamous cell carcinoma (CSCC).
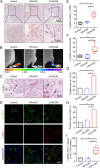
Methods: Immunofluorescent staining of α-SMA, LYVE-1 and DAPI were examined in primary tumour samples obtained from 57 CSCC patients. Assessment of cytokines secreted by CAFs and normal fibroblasts (NFs) was performed using human cytokine antibody arrays. The phenotype of EndoMT in lymphatic endothelial cells (LECs), gene expression levels, protein secretion and activity of signaling pathways were measured by real-time RT-PCR, ELISA or western blotting. The function of lymphatic endothelial monolayers was examined by transwell, tube formation assay, transendothelial migration assay in vitro. Lymphatic metastasis was measured using popliteal lymph node metastasis model. Furthermore, association between PAI-1 expression and EndoMT in CSCC was analyzed by immunohistochemistry. The Cancer Genome Atlas (TCGA) databases was used to assess the association of PAI-1 with survival rate in CSCC.
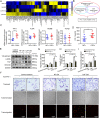
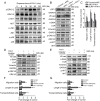
Results: CAF-derived PAI-1 promoted the EndoMT of LECs in CSCC. LECs undergoing EndoMT could initiate tumour neolymphangiogenesis that facilitated cancer cell intravasation/extravasation, which in turn promoted lymphatic metastasis in CSCC. Mechanistically, PAI-1 activated the AKT/ERK1/2 pathways by directly interacting with low-density lipoprotein receptor-related protein (LRP1), thereby leading to elevated EndoMT activity in LECs. Blockade of PAI-1 or inhibition of LRP1/AKT/ERK1/2 abrogated EndoMT and consequently attenuated CAF-induced tumour neolymphangiogenesis. Furthermore, clinical data revealed that increased PAI-1 levels positively correlated with EndoMT activity and poor prognosis in CSCC patients.
Conclusion: Our data indicate that CAF-derived PAI-1 acts as an important neolymphangiogenesis-initiating molecular during CSCC progression through modulating the EndoMT of LECs, resulting in promotion of metastasis ability in primary site. PAI-1 could serve as an effective prognostic biomarker and therapeutic target for CSCC metastasis.
Keywords: Cancer-associated fibroblast; Cervical squamous cell carcinoma; Endothelial-mesenchymal transition; Lymphatic metastasis; PAI-1.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Bieniasz-Krzywiec P, Martin-Perez R, Ehling M, Garcia-Caballero M, Pinioti S, Pretto S, Kroes R, Aldeni C, Di Matteo M, Prenen H, Tribulatti MV, Campetella O, Smeets A, Noel A, Floris G, Van Ginderachter JA, Mazzone M. Podoplanin-expressing macrophages promote lymphangiogenesis and lymphoinvasion in breast cancer. Cell Metab. 2019;30:917–936 e10. doi: 10.1016/j.cmet.2019.07.015. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

