Extracorporeal hemoadsorption therapy as a potential therapeutic option for rapid removal of Apixaban in high risk-surgical patients: a case report
- PMID: 37415195
- PMCID: PMC10327312
- DOI: 10.1186/s13256-023-03949-3
Extracorporeal hemoadsorption therapy as a potential therapeutic option for rapid removal of Apixaban in high risk-surgical patients: a case report
Abstract
Background: Apixaban is a non-vitamin K antagonist oral anticoagulant (NOACs) recently emerged as an effective alternative to conventional vitamin K antagonists (VKAs) in the treatment of several thromboembolic disorders. However, in case of overdose or in patients requiring emergency surgery there is a high bleeding rate and severe adverse side effects due to the absence of an antidote. There is promising data from in vitro and clinical studies, that certain antithrombotic agents (that is Rivaroxaban and Ticagrelor) have been successfully removed by the extracorporeal hemoadsorption therapy CytoSorb. Here, we present the case of a patient successfully treated with CytoSorb as a kind of antidote to enable emergency surgery for bilateral nephrostomy.
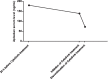
Case presentation: A 82-year-old Caucasian man was admitted to the Emergency Room with acute kidney injury (AKI) in the context of severe bilateral hydroureteronephrosis. The patient's medical history included chronic obstructive pulmonary disease, arterial hypertension, atrial fibrillation (anticoagulated with Apixaban) and a locally advanced prostate adenocarcinoma treated with trans-ureteral resection of the bladder and radiotherapy in the previous months. The indication for a bilateral nephrostomy could not be considered immediately given the major bleeding risk due to Apixaban, which was discontinued and replaced with calciparin. After 36 hours of continuous renal replacement therapy (CRRT), the Apixaban blood level was still elevated and it was decided to install CytoSorb into the running CRRT to accelerate the drug clearance. After 2 hours 30 minutes, there was good reduction of Apixaban from 139 to 72 ng/ml (reduction rate of 48.2%) registered, and this allowed for an easy placement of bilateral nephrostomies without complications. Four days after surgery renal function parameters further normalized, the patient did not require additional dialysis treatments and Apixaban therapy was prescribed again once the patient returned home.
Conclusions: In this case we report the findings of a patient with post-renal AKI requiring emergency nephrostomy placement while on chronic anticoagulation with Apixaban therapy. Combined treatment with CRRT and CytoSorb was associated with the rapid and effective removal of Apixaban allowing for prompt and urgent surgery while simultaneously ensuring the low risk of bleeding as well as an uneventful post-operative course.
Keywords: Apixaban; CytoSorb; Hemoadsorption.
© 2023. The Author(s).
Conflict of interest statement
The authors have no competing interests to declare.
Figures
References
-
- Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17:1467–1507. doi: 10.1093/europace/euv309. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical