EP3 signaling is decoupled from the regulation of glucose-stimulated insulin secretion in β-cells compensating for obesity and insulin resistance
- PMID: 37415404
- PMCID: PMC10332234
- DOI: 10.1080/19382014.2023.2223327
EP3 signaling is decoupled from the regulation of glucose-stimulated insulin secretion in β-cells compensating for obesity and insulin resistance
Abstract
Of the β-cell signaling pathways altered by obesity and insulin resistance, some are adaptive while others contribute to β-cell failure. Two critical second messengers are Ca2+ and cAMP, which control the timing and amplitude of insulin secretion. Previous work has shown the importance of the cAMP-inhibitory Prostaglandin EP3 receptor (EP3) in mediating the β-cell dysfunction of type 2 diabetes (T2D). Here, we used three groups of C57BL/6J mice as a model of the progression from metabolic health to T2D: wildtype, normoglycemic LeptinOb (NGOB), and hyperglycemic LeptinOb (HGOB). Robust increases in β-cell cAMP and insulin secretion were observed in NGOB islets as compared to wildtype controls; an effect lost in HGOB islets, which exhibited reduced β-cell cAMP and insulin secretion despite increased glucose-dependent Ca2+ influx. An EP3 antagonist had no effect on β-cell cAMP or Ca2+ oscillations, demonstrating agonist-independent EP3 signaling. Finally, using sulprostone to hyperactivate EP3 signaling, we found EP3-dependent suppression of β-cell cAMP and Ca2+ duty cycle effectively reduces insulin secretion in HGOB islets, while having no impact insulin secretion on NGOB islets, despite similar and robust effects on cAMP levels and Ca2+ duty cycle. Finally, increased cAMP levels in NGOB islets are consistent with increased recruitment of the small G protein, Rap1GAP, to the plasma membrane, sequestering the EP3 effector, Gɑz, from inhibition of adenylyl cyclase. Taken together, these results suggest that rewiring of EP3 receptor-dependent cAMP signaling contributes to the progressive changes in β cell function observed in the LeptinOb model of diabetes.
Keywords: Diabetes; EP3 receptor; Gɑz; Rap1gap; cAMP; hyperglycemia; insulin secretion; prostaglandins; β-cell.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


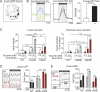


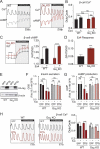

References
-
- Kimple ME, Keller MP, Rabaglia MR, Pasker RL, Neuman JC, Truchan NA, Brar HK, Attie AD. Prostaglandin E2 receptor, EP3, is induced in diabetic islets and negatively regulates glucose- and hormone-stimulated insulin secretion. Diabetes. 2013;62(6):1904–1912. doi:10.2337/db12-0769. - DOI - PMC - PubMed
-
- Parazzoli S, Harmon JS, Vallerie SN, Zhang T, Zhou H, Robertson RP. Cyclooxygenase-2, not microsomal prostaglandin E Synthase-1, is the mechanism for interleukin-1β-induced prostaglandin E2 production and inhibition of insulin secretion in pancreatic islets*. Journal Of Biological Chemistryquery. 2012;287(38):32246–32253. doi:10.1074/jbc.M112.364612. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
