The female upper reproductive tract harbors endogenous microbial profiles
- PMID: 37415669
- PMCID: PMC10321600
- DOI: 10.3389/fendo.2023.1096050
The female upper reproductive tract harbors endogenous microbial profiles
Abstract
Introduction: The female reproductive tract harbours unique microbial communities (known as microbiota) which have been associated with reproductive functions in health and disease. While endometrial microbiome studies have shown that the uterus possesses higher bacterial diversity and richness compared to the vagina, the knowledge regarding the composition of the Fallopian tubes (FT) is lacking, especially in fertile women without any underlying conditions.
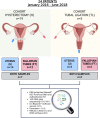
Methods: To address this gap, our study included 19 patients who underwent abdominal hysterectomy for benign uterine pathology, and 5 women who underwent tubal ligation as a permanent contraceptive method at Hospital Clínico Universitario Virgen de la Arrixaca (HCUVA). We analyzed the microbiome of samples collected from the FT and endometrium using 16S rRNA gene sequencing.
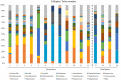
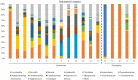
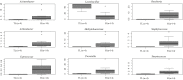
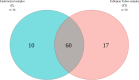
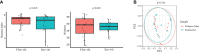
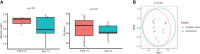
Results: Our findings revealed distinct microbiome profiles in the endometrial and FT samples, indicating that the upper reproductive tract harbors an endogenous microbiome. However, these two sites also shared some similarities, with 69% of the detected taxa Being common to both. Interestingly, we identified seventeen bacterial taxa exclusively present in the FT samples, including the genera Enhydrobacter, Granulicatella, Haemophilus, Rhizobium, Alistipes, and Paracoccus, among others. On the other hand, 10 bacterial taxa were only found in the endometrium, including the genera Klebsiella, Olsenella, Oscillibacter and Veillonella (FDR <0.05). Furthermore, our study highlighted the influence of the endometrial collection method on the findings. Samples obtained transcervically showed a dominance of the genus Lactobacillus, which may indicate potential vaginal contamination. In contrast, uterine samples obtained through hysterescopy revealed higher abundance of the genera Acinetobacter, Arthrobacter, Coprococcus, Methylobacterium, Prevotella, Roseburia, Staphylococcus, and Streptococcus.
Discussion: Although the upper reproductive tract appears to have a low microbial biomass, our results suggest that the endometrial and FT microbiome is unique to each individual. In fact, samples obtained from the same individual showed more microbial similarity between the endometrium and FT compared to samples from different women. Understanding the composition of the female upper reproductive microbiome provides valuable insights into the natural microenvironment where processes such as oocyte fertilization, embryo development and implantation occur. This knowledge can improve in vitro fertilization and embryo culture conditions for the treatment of infertility.
Keywords: 16S rRNA gene; endometrium; fallopian tubes; microbes; microbiome; microbiota; upper reproductive tract.
Copyright © 2023 Canha-Gouveia, Pérez-Prieto, Rodríguez, Escamez, Leonés-Baños, Salas-Espejo, Prieto-Sánchez, Sánchez-Ferrer, Coy and Altmäe.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Evidence that the endometrial microbiota has an effect on implantation success or failure.Am J Obstet Gynecol. 2016 Dec;215(6):684-703. doi: 10.1016/j.ajog.2016.09.075. Epub 2016 Oct 4. Am J Obstet Gynecol. 2016. PMID: 27717732
-
Microbial signatures and continuum in endometrial cancer and benign patients.Microbiome. 2024 Jul 1;12(1):118. doi: 10.1186/s40168-024-01821-0. Microbiome. 2024. PMID: 38951935 Free PMC article.
-
The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases.Nat Commun. 2017 Oct 17;8(1):875. doi: 10.1038/s41467-017-00901-0. Nat Commun. 2017. PMID: 29042534 Free PMC article.
-
The Role of the Vaginal and Endometrial Microbiomes in Infertility and Their Impact on Pregnancy Outcomes in Light of Recent Literature.Int J Mol Sci. 2024 Dec 9;25(23):13227. doi: 10.3390/ijms252313227. Int J Mol Sci. 2024. PMID: 39684937 Free PMC article. Review.
-
Relevance of assessing the uterine microbiota in infertility.Fertil Steril. 2018 Aug;110(3):337-343. doi: 10.1016/j.fertnstert.2018.04.041. Fertil Steril. 2018. PMID: 30098680 Review.
Cited by
-
Exploring Immunome and Microbiome Interplay in Reproductive Health: Current Knowledge, Challenges, and Novel Diagnostic Tools.Semin Reprod Med. 2023 Sep;41(5):172-189. doi: 10.1055/s-0043-1778017. Epub 2024 Jan 23. Semin Reprod Med. 2023. PMID: 38262441 Free PMC article. Review.
-
Gynecological Cancers and Microbiota Dynamics: Insights into Pathogenesis and Therapy.Int J Mol Sci. 2024 Feb 13;25(4):2237. doi: 10.3390/ijms25042237. Int J Mol Sci. 2024. PMID: 38396914 Free PMC article. Review.
-
Advanced Technologies for Studying Microbiome-Female Reproductive Tract Interactions: Organoids, Organoids-on-a-Chip, and Beyond.Semin Reprod Med. 2023 Sep;41(5):160-171. doi: 10.1055/s-0043-1778067. Epub 2024 Jan 23. Semin Reprod Med. 2023. PMID: 38262440 Free PMC article. Review.
-
Deciphering the role of female reproductive tract microbiome in reproductive health: a review.Front Cell Infect Microbiol. 2024 Mar 18;14:1351540. doi: 10.3389/fcimb.2024.1351540. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38562966 Free PMC article. Review.
-
Comparison of RNA- and DNA-based 16S amplicon sequencing to find the optimal approach for the analysis of the uterine microbiome.Sci Rep. 2025 May 16;15(1):17037. doi: 10.1038/s41598-025-00969-5. Sci Rep. 2025. PMID: 40379732 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

