Integrating qualitative interviews in drug development and the use of qualitative evidence in product labelling and health technology assessments: a review
- PMID: 37415771
- PMCID: PMC10322192
- DOI: 10.3389/fmed.2023.1197529
Integrating qualitative interviews in drug development and the use of qualitative evidence in product labelling and health technology assessments: a review
Abstract
Objective: Including qualitative research in clinical trial design is an innovative approach to understanding patients' perspective and incorporate the patient's voice in all stages of drug development and evaluation. This review aims to explore current practices, lessons learned from the literature, as well as how qualitative interviews are considered by health authorities for marketing authorization and reimbursement.
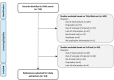
Methods: A targeted literature review of Medline and Embase databases was conducted in February 2022 to identify publications on qualitative methods embedded in clinical trial of pharmaceutical products. An additional search of guidelines and labeling claims of approved products regarding qualitative research was performed in various sources of grey literature.
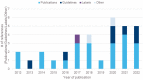
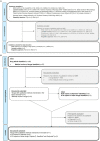
Results: From the 24 publications and nine documents reviewed, we identified the research questions addressed with qualitative methods during clinical trials (e.g., change in quality of life, symptoms assessment, treatment benefit), preferred data collection methods (e.g., interviews), and data collection points (e.g., baseline and exit interviews). Moreover, the data from labels and HTAs demonstrate that qualitative data can play an important role in approval processes.
Conclusion: The use of in-trial interviews is still emerging and is not yet common practice. Although the industry, scientific community, regulatory agencies and HTAs are showing an increasing interest in the use of evidence generated via in-trial interviews, guidance from regulators and HTAs would be helpful. Developing new methods and technologies to address the common challenges for such interviews is key to progress.
Keywords: chronic disease; clinical trial; interview; mixed-method approaches; patient-focused drug development; qualitative research and analysis.
Copyright © 2023 Michel, Kamudoni, Marrel, Adiutori, Desvignes-Gleizes, Lanar, Schache, Spies and Park.
Conflict of interest statement
A-SM, AM, RA, CD-G, and SL are employees of ICON. PS is an employee of LAIFE REPLY. PK is an employee of Merck KgaA. JP and ES are employees of EMD Serono (a company of Merck KgaA). ICON and LAIFE REPLY were contracted with Merck KgaA as consultants on patient-centered research activities.
Figures
Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
Modelling approaches for histology-independent cancer drugs to inform NICE appraisals: a systematic review and decision-framework.Health Technol Assess. 2021 Dec;25(76):1-228. doi: 10.3310/hta25760. Health Technol Assess. 2021. PMID: 34990339
-
Methods for the comparative evaluation of pharmaceuticals.GMS Health Technol Assess. 2005 Nov 15;1:Doc09. GMS Health Technol Assess. 2005. PMID: 21289930 Free PMC article.
-
Use of Patient Preference Information in Benefit-Risk Assessment, Health Technology Assessment, and Pricing and Reimbursement Decisions: A Systematic Literature Review of Attempts and Initiatives.Front Med (Lausanne). 2020 Oct 26;7:543046. doi: 10.3389/fmed.2020.543046. eCollection 2020. Front Med (Lausanne). 2020. PMID: 33195294 Free PMC article.
Cited by
-
Patient Experience of Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease: Qualitative Patient and Clinician Interviews Informing the Development of a Conceptual Model.Neurol Ther. 2025 Aug;14(4):1569-1587. doi: 10.1007/s40120-025-00770-6. Epub 2025 Jun 12. Neurol Ther. 2025. PMID: 40506564 Free PMC article.
-
Exit Interviews to Understand Meaningful Change from the Patient Perspective in a Clinical Study of Dupilumab for the Treatment of Chronic Inducible Cold Urticaria.Patient. 2025 Jul 22. doi: 10.1007/s40271-025-00754-6. Online ahead of print. Patient. 2025. PMID: 40696090
-
Qualitative In-trial Interviews: Methods, Challenges, and Best Practice.Patient. 2025 May;18(3):199-209. doi: 10.1007/s40271-024-00726-2. Epub 2025 Feb 3. Patient. 2025. PMID: 39899208
-
Patient-Reported Meaningful Change in Symptoms and Impacts of Paroxysmal Nocturnal Hemoglobinuria (PNH) in Three Phase III Clinical Trials of Iptacopan.Patient. 2025 Jul 22. doi: 10.1007/s40271-025-00755-5. Online ahead of print. Patient. 2025. PMID: 40694294
References
-
- FDA . Patient-focused drug development: methods to identify what is important to patients. Maryland, US: Food and Drug Administration; (2022).
-
- FDA . Patient-reported outcome measures: use in medical product development to support labeling claims. Maryland, US: Food and Drug Administration; (2009).
-
- Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E, et al. . Content validity--establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1--eliciting concepts for a new PRO instrument. Value Health. (2011) 14:967–77. doi: 10.1016/j.jval.2011.06.014 - DOI - PubMed
-
- Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E, et al. . Content validity--establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 2--assessing respondent understanding. Value Health. (2011) 14:978–88. doi: 10.1016/j.jval.2011.06.013 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

