Assessment of mortality-related risk factors and effective antimicrobial regimens for treatment of bloodstream infections caused by carbapenem-resistant Pseudomonas aeruginosa in patients with hematological diseases
- PMID: 37415825
- PMCID: PMC10320591
- DOI: 10.3389/fcimb.2023.1156651
Assessment of mortality-related risk factors and effective antimicrobial regimens for treatment of bloodstream infections caused by carbapenem-resistant Pseudomonas aeruginosa in patients with hematological diseases
Abstract
Background: Infections caused by carbapenem-resistant Pseudomonas aeruginosa (CRPA) are related to higher mortality. The objective of this study was to explore clinical outcomes of CRPA bacteremia, identify risk factors and also, compare the efficacy of traditional and novel antibiotic regimens.
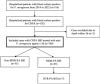
Methods: This retrospective study was conducted at a blood diseases hospital in China. The study included hematological patients who were diagnosed with CRPA bacteremia between January 2014 and August 2022. The primary endpoint was all-cause mortality at day 30. Secondary endpoints included 7-day and 30-day clinical cure. Multivariable Cox regression analysis was employed to identify mortality-related risk factors.
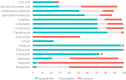
Results: 100 patients infected with CRPA bacteremia were included and 29 patients accepted allogenic-hematopoietic stem cell transplantation. 24 received ceftazidime-avibactam (CAZ-AVI)-based therapy and 76 received other traditional antibiotics. 30-day mortality was 21.0%. Multivariable cox regression analysis showed neutropenia >7 days after bloodstream infections (BSI) (P=0.030, HR: 4.068, 95%CI: 1.146~14.434), higher Pitt bacteremia score (P<0.001, HR:1.824, 95%CI: 1.322~2.517), higher Charlson comorbidity index (P=0.01, HR: 1.613, 95%CI: 1.124~2.315) and bacteremia due to multidrug-resistant Pseudomonas aeruginosa (MDR-PA) (P=0.024, HR:3.086, 95%CI: 1.163~8.197) were identified as independent risk factors of 30-day mortality. After controlling for confounders, an additional multivariable cox regression analysis revealed definitive regimens containing CAZ-AVI were associated with lower mortality in CRPA bacteremia (P=0.016, HR: 0.150, 95%CI: 0.032~0.702), as well as in MDR-PA bacteremia (P=0.019, HR: 0.119, 95%CI: 0.020~0.709).
Conclusions: For patients with hematological diseases and CRPA bacteremia, 30-day mortality rate was 21.0% (21/100). Neutropenia >7 days after BSI, higher Pitt bacteremia score, higher Charlson comorbidity index and bacteremia due to MDR-PA increased 30-day mortality. CAZ-AVI-based regimens were effective alternatives for bacteremia due to CRPA or MDR-PA.
Keywords: bacteremia; carbapenem-resistant Pseudomonas aeruginosa; ceftazidime-avibactam; hematological diseases; multidrug-resistant Pseudomonas aeruginosa.
Copyright © 2023 Zhen, Zhao, Chen, Zhang, Wang, Jiang, Zhang, Mi, Zhu, Han, Xiao, Wang and Feng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Ceftazidime-avibactam in the treatment of bacteremia due to carbapenem-resistant gram-negative bacteria in hematological patients: Experience in a single center.J Infect Chemother. 2024 Jul;30(7):608-615. doi: 10.1016/j.jiac.2024.01.007. Epub 2024 Jan 11. J Infect Chemother. 2024. PMID: 38215820
-
The efficacy and safety of ceftazidime/avibactam or polymyxin B based regimens for carbapenem-resistant Pseudomonas aeruginosa infection: a multicenter real-world and propensity score-matched study.Front Pharmacol. 2025 Mar 31;16:1533952. doi: 10.3389/fphar.2025.1533952. eCollection 2025. Front Pharmacol. 2025. PMID: 40230702 Free PMC article.
-
Clinical efficacy of ceftazidime/avibactam combination therapy for severe hospital-acquired pulmonary infections caused by carbapenem-resistant and difficult-to-treat Pseudomonas aeruginosa.Int J Antimicrob Agents. 2024 Jan;63(1):107021. doi: 10.1016/j.ijantimicag.2023.107021. Epub 2023 Oct 27. Int J Antimicrob Agents. 2024. PMID: 37890733
-
Efficacy and Safety of Ceftazidime-Avibactam for the Treatment of Carbapenem-Resistant Enterobacterales Bloodstream Infection: a Systematic Review and Meta-Analysis.Microbiol Spectr. 2022 Apr 27;10(2):e0260321. doi: 10.1128/spectrum.02603-21. Epub 2022 Apr 4. Microbiol Spectr. 2022. PMID: 35377233 Free PMC article.
-
Update of clinical application in ceftazidime-avibactam for multidrug-resistant Gram-negative bacteria infections.Infection. 2022 Dec;50(6):1409-1423. doi: 10.1007/s15010-022-01876-x. Epub 2022 Jul 4. Infection. 2022. PMID: 35781869 Review.
Cited by
-
Rapid diagnosis of Aspergillus flavus infection in acute very severe aplastic anemia with metagenomic next-generation sequencing: a case report and literature review.Front Med (Lausanne). 2024 Sep 23;11:1413964. doi: 10.3389/fmed.2024.1413964. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39376649 Free PMC article.
-
Performance evaluation of four antibiotics using the BD Phoenix™ NMIC-413 antimicrobial susceptibility testing panel for carbapenem-resistant Enterobacteriaceae and carbapenem-resistant Pseudomonas aeruginosa.Front Microbiol. 2025 Jul 4;16:1593674. doi: 10.3389/fmicb.2025.1593674. eCollection 2025. Front Microbiol. 2025. PMID: 40687849 Free PMC article.
-
Three prolonged outbreaks of metallo-β-lactamase-producing Pseudomonas aeruginosa in an Upper Austrian hospital, 2017-2023.Microbiol Spectr. 2024 Oct 3;12(10):e0074024. doi: 10.1128/spectrum.00740-24. Epub 2024 Aug 20. Microbiol Spectr. 2024. PMID: 39162508 Free PMC article.
-
Adult acute leukemia patients with gram-negative bacteria bloodstream infection: Risk factors and outcomes of antibiotic-resistant bacteria.Ann Hematol. 2024 Oct;103(10):4021-4031. doi: 10.1007/s00277-024-05866-x. Epub 2024 Jul 3. Ann Hematol. 2024. PMID: 38958702 Free PMC article.
-
Ceftazidime-avibactam combination therapy versus monotherapy for treating carbapenem-resistant gram-negative infection: a systemic review and meta-analysis.Infection. 2024 Oct;52(5):2029-2042. doi: 10.1007/s15010-024-02277-y. Epub 2024 May 13. Infection. 2024. PMID: 38739208
References
-
- Bergas A., Albasanz-Puig A., Fernández-Cruz A., Machado M., Novo A., van Duin D., et al. . (2022). Real-life use of Ceftolozane/Tazobactam for the treatment of bloodstream infection due to pseudomonas aeruginosa in neutropenic hematologic patients: a matched control study (ZENITH study). Microbiol. Spectrum. 10 (3), e0229221. doi: 10.1128/spectrum.02292-21 - DOI - PMC - PubMed
-
- Buehrle D. J., Shields R. K., Clarke L. G., Potoski B. A., Clancy C. J., Nguyen M. H. (2017). Carbapenem-resistant pseudomonas aeruginosa bacteremia: risk factors for mortality and microbiologic treatment failure. Antimicrob. Agents Chemother. 61 (1), e01243–16. doi: 10.1128/aac.01243-16 - DOI - PMC - PubMed
-
- Bulik C. C., Christensen H., Li P., Sutherland C. A., Nicolau D. P., Kuti J. L. (2010). Comparison of the activity of a human simulated, high-dose, prolonged infusion of meropenem against klebsiella pneumoniae producing the KPC carbapenemase versus that against pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 54 (2), 804–810. doi: 10.1128/aac.01190-09 - DOI - PMC - PubMed
-
- Chen Z., Xu Z., Wu H., Chen L., Gao S., Chen Y. (2019). The impact of carbapenem-resistant pseudomonas aeruginosa on clinical and economic outcomes in a Chinese tertiary care hospital: a propensity score-matched analysis. Am. J. Infect. Control. 47 (6), 677–682. doi: 10.1016/j.ajic.2018.10.025 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

