UV light-induced spatial loss of sialic acid capping using a photoactivatable sialyltransferase inhibitor
- PMID: 37415865
- PMCID: PMC10320844
- DOI: 10.1039/d3cb00006k
UV light-induced spatial loss of sialic acid capping using a photoactivatable sialyltransferase inhibitor
Abstract
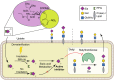
Sialic acids cap glycans displayed on mammalian glycoproteins and glycolipids and mediate many glycan-receptor interactions. Sialoglycans play a role in diseases such as cancer and infections where they facilitate immune evasion and metastasis or serve as cellular receptors for viruses, respectively. Strategies that specifically interfere with cellular sialoglycan biosynthesis, such as sialic acid mimetics that act as metabolic sialyltransferase inhibitors, enable research into the diverse biological functions of sialoglycans. Sialylation inhibitors are also emerging as potential therapeutics for cancer, infection, and other diseases. However, sialoglycans serve many important biological functions and systemic inhibition of sialoglycan biosynthesis can have adverse effects. To enable local and inducible inhibition of sialylation, we have synthesized and characterized a caged sialyltransferase inhibitor that can be selectively activated with UV-light. A photolabile protecting group was conjugated to a known sialyltransferase inhibitor (P-SiaFNEtoc). This yielded a photoactivatable inhibitor, UV-SiaFNEtoc, that remained inactive in human cell cultures and was readily activated through radiation with 365 nm UV light. Direct and short radiation of a human embryonic kidney (HEK293) cell monolayer was well-tolerated and resulted in photoactivation of the inhibitor and subsequent spatial restricted synthesis of asialoglycans. The developed photocaged sialic acid mimetic holds the potential to locally hinder the synthesis of sialoglycans through focused treatment with UV light and may be applied to bypass the adverse effects related to systemic loss of sialylation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
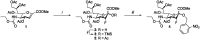



Similar articles
-
Putting a cap on the glycome: Dissecting human sialyltransferase functions.Carbohydr Res. 2024 Oct;544:109242. doi: 10.1016/j.carres.2024.109242. Epub 2024 Aug 13. Carbohydr Res. 2024. PMID: 39167930 Review.
-
Insights into the Role of Sialylation in Cancer Metastasis, Immunity, and Therapeutic Opportunity.Cancers (Basel). 2022 Nov 26;14(23):5840. doi: 10.3390/cancers14235840. Cancers (Basel). 2022. PMID: 36497322 Free PMC article. Review.
-
Enzymatic basis for N-glycan sialylation: structure of rat α2,6-sialyltransferase (ST6GAL1) reveals conserved and unique features for glycan sialylation.J Biol Chem. 2013 Nov 29;288(48):34680-98. doi: 10.1074/jbc.M113.519041. Epub 2013 Oct 23. J Biol Chem. 2013. PMID: 24155237 Free PMC article.
-
Targeting aberrant sialylation in cancer cells using a fluorinated sialic acid analog impairs adhesion, migration, and in vivo tumor growth.Mol Cancer Ther. 2013 Oct;12(10):1935-46. doi: 10.1158/1535-7163.MCT-13-0279. Epub 2013 Aug 23. Mol Cancer Ther. 2013. PMID: 23974695
-
Biological Functions and Analytical Strategies of Sialic Acids in Tumor.Cells. 2020 Jan 22;9(2):273. doi: 10.3390/cells9020273. Cells. 2020. PMID: 31979120 Free PMC article. Review.
References
-
- Varki A., Schnaar R. L. and Schauer R., Sialic Acids and Other Nonulosonic Acids. in Essentials of Glycobiology [Internet], ed. A. Varki, R.Cummings D., Esko J. D., Stanley P., Hart G. W., Aebi M., Darvill A. G., Kinoshita T., Packer N. H., Prestegard J. H., Schnaar R. L. and Seeberger P. H., Cold Spring Harbor Laboratory Press, Cold Spring Harbor; (NY: ), 3rd edn, 2015–2017, ch. 15
LinkOut - more resources
Full Text Sources
Miscellaneous

