Bilateral transcranial direct-current stimulation promotes migration of subventricular zone-derived neuroblasts toward ischemic brain
- PMID: 37415929
- PMCID: PMC10320846
- DOI: 10.1096/fba.2023-00017
Bilateral transcranial direct-current stimulation promotes migration of subventricular zone-derived neuroblasts toward ischemic brain
Abstract
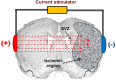
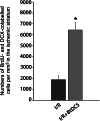
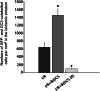
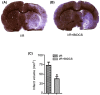
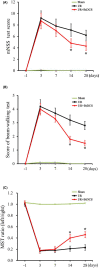
Ischemic insult stimulates proliferation of neural stem cells (NSCs) in the subventricular zone (SVZ) after stroke. However, only a fraction of NSC-derived neuroblasts from SVZ migrate toward poststroke brain region. We have previously reported that direct-current stimulation guides NSC migration toward the cathode in vitro. Accordingly, we set up a new method of transcranial direct-current stimulation (tDCS), in which the cathodal electrode is placed on the ischemic hemisphere and anodal electrode on the contralateral hemisphere of rats subjected to ischemia-reperfusion injury. We show that the application of this bilateral tDCS (BtDCS) promotes the migration of NSC-derived neuroblasts from SVZ toward the cathode direction into poststroke striatum. Reversing the position of the electrodes blocks the effect of BtDCS on the migration of neuroblasts from SVZ. BtDCS protects against neuronal death and improves the functional recovery of stroke animals. Thus, the migration of NSC-derived neuroblasts from SVZ toward poststroke brain region contributes to the effect of BtDCS against ischemia-induced neuronal death, supporting a potential development of noninvasive BtDCS as an endogenous neurogenesis-based stroke therapy.
Keywords: ischemic stroke; neural stem cells; stem cell migration; subventricular zone; transcranial direct‐current stimulation.
© 2023 The Authors. FASEB BioAdvances published by Wiley Periodicals LLC on behalf of The Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Cathodal bilateral transcranial direct-current stimulation regulates selenium to confer neuroprotection after rat cerebral ischaemia-reperfusion injury.J Physiol. 2024 Mar;602(6):1175-1197. doi: 10.1113/JP285806. Epub 2024 Mar 3. J Physiol. 2024. PMID: 38431908
-
Neuroblast division during migration toward the ischemic striatum: a study of dynamic migratory and proliferative characteristics of neuroblasts from the subventricular zone.J Neurosci. 2007 Mar 21;27(12):3157-62. doi: 10.1523/JNEUROSCI.4969-06.2007. J Neurosci. 2007. PMID: 17376977 Free PMC article.
-
Cerebral dopamine neurotrophic factor increases proliferation, Migration and differentiation of subventricular zone neuroblasts in photothrombotic stroke model of mouse.J Stroke Cerebrovasc Dis. 2022 Nov;31(11):106725. doi: 10.1016/j.jstrokecerebrovasdis.2022.106725. Epub 2022 Sep 15. J Stroke Cerebrovasc Dis. 2022. PMID: 36116218
-
Regeneration using endogenous neural stem cells following neonatal brain injury.Pediatr Int. 2021 Jan;63(1):13-21. doi: 10.1111/ped.14368. Epub 2021 Jan 11. Pediatr Int. 2021. PMID: 32609915 Review.
-
The Adult Ventricular-Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) Neurogenesis.Cold Spring Harb Perspect Biol. 2016 May 2;8(5):a018820. doi: 10.1101/cshperspect.a018820. Cold Spring Harb Perspect Biol. 2016. PMID: 27048191 Free PMC article. Review.
Cited by
-
Effect of Dual-Site Non-Invasive Brain Stimulation on Upper-Limb Function After Stroke: A Systematic Review and Meta-Analysis.Brain Behav. 2024 Nov;14(11):e70145. doi: 10.1002/brb3.70145. Brain Behav. 2024. PMID: 39508474 Free PMC article.
-
Molecular Changes in the Ischemic Brain as Non-Invasive Brain Stimulation Targets-TMS and tDCS Mechanisms, Therapeutic Challenges, and Combination Therapies.Biomedicines. 2024 Jul 13;12(7):1560. doi: 10.3390/biomedicines12071560. Biomedicines. 2024. PMID: 39062133 Free PMC article. Review.
-
Bioelectricity in dental medicine: a narrative review.Biomed Eng Online. 2024 Jan 3;23(1):3. doi: 10.1186/s12938-023-01189-6. Biomed Eng Online. 2024. PMID: 38172866 Free PMC article. Review.
References
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963‐970. - PubMed
-
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33‐41. - PubMed
-
- Felling RJ, Levison SW. Enhanced Neurogenesis following stroke. J Neurosci Res. 2003;73:277‐283. - PubMed
LinkOut - more resources
Full Text Sources
