Elevated follicular cortisone level is a negative predictor of clinical pregnancy in women undergoing fresh embryo transfer
- PMID: 37415947
- PMCID: PMC10320308
- DOI: 10.1016/j.heliyon.2023.e17492
Elevated follicular cortisone level is a negative predictor of clinical pregnancy in women undergoing fresh embryo transfer
Abstract
Background: Although numerous studies have investigated the potential correlation between follicular fluid (FF) steroid concentrations and in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) outcomes, few have accounted for the effect of controlled ovarian hyperstimulation regimes on FF steroid concentrations.
Objective: To comprehensively compare follicular steroid concentrations between women stimulated with gonadotropin-releasing hormone agonist (GnRHa) and antagonist (GnRHant) protocols and to explore the associations between FF steroid concentrations and IVF/ICSI outcomes.
Methods: A total of 295 infertile women undergoing IVF/ICSI from January 2018 to May 2020 were enrolled. Eighty-four and 211 women received GnRHa and GnRHant protocols, respectively. Seventeen steroids in FF were quantified by liquid chromatography tandem mass spectrometry (LC-MS/MS), and the correlation of follicular steroids with clinical pregnancy was explored.
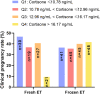
Results: Follicular steroid concentrations were similar between the GnRHa and GnRHant groups. Follicular cortisone levels were adversely associated with clinical pregnancy in fresh embryo transfers. Receiver operating characteristic (ROC) analysis revealed an area under the ROC curve (AUC) of 0.639 (95% confidence interval = 0.527-0.751, p = 0.025) for predicting non-pregnancy, with an optimal cutoff value of 15.81 ng/mL (sensitivity = 33.3%, specificity = 94.1%). Women with FF cortisone concentrations ≥15.81 ng/mL were fifty times less likely to achieve clinical pregnancy in fresh embryo transfers than those with FF cortisone levels below this threshold (adjusted OR = 0.019, 95% confidence interval = 0.002-0.207, p = 0.001) after adjusting for age, body mass index, baseline serum progesterone levels, serum levels of luteinizing hormone, estradiol and progesterone on human chorionic gonadotropin day, ovarian stimulation protocols, and the number of transferred embryos.
Conclusions: There was no significant difference in intrafollicular steroid levels between GnRHa and GnRHant protocols, and intrafollicular cortisone level ≥15.81 ng/mL was found to be a strong negative predictor of clinical pregnancy in fresh embryo transfers with high specificity.
Keywords: Assisted reproduction technology; Clinical pregnancy; Cortisone; Follicular fluid; LC-MS/MS.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Oocyte matched follicular fluid anti-Müllerian hormone is an excellent predictor of live birth after fresh single embryo transfer.Hum Reprod. 2019 Nov 1;34(11):2244-2253. doi: 10.1093/humrep/dez186. Hum Reprod. 2019. PMID: 31725884
-
Effect of progesterone/estradiol ratio on pregnancy outcome of patients with high trigger-day progesterone levels undergoing gonadotropin-releasing hormone antagonist intracytoplasmic sperm injection cycles: a retrospective cohort study.J Obstet Gynaecol. 2019 Feb;39(2):157-163. doi: 10.1080/01443615.2018.1504204. Epub 2018 Oct 3. J Obstet Gynaecol. 2019. PMID: 30280612
-
Relationship between ovarian cortisol:cortisone ratios and the clinical outcome of in vitro fertilization and embryo transfer (IVF-ET).Clin Endocrinol (Oxf). 1999 Nov;51(5):535-40. doi: 10.1046/j.1365-2265.1999.00892.x. Clin Endocrinol (Oxf). 1999. PMID: 10594513
-
Association Between Progesterone Elevation on the Day of Human Chronic Gonadotropin Trigger and Pregnancy Outcomes After Fresh Embryo Transfer in In Vitro Fertilization/Intracytoplasmic Sperm Injection Cycles.Front Endocrinol (Lausanne). 2018 Apr 26;9:201. doi: 10.3389/fendo.2018.00201. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29755412 Free PMC article. Review.
-
Conventional GnRH antagonist protocols versus long GnRH agonist protocol in IVF/ICSI cycles of polycystic ovary syndrome women: a systematic review and meta-analysis.Sci Rep. 2022 Mar 15;12(1):4456. doi: 10.1038/s41598-022-08400-z. Sci Rep. 2022. PMID: 35292717 Free PMC article.
References
-
- Piccinni M.P., Vicenti R., Logiodice F., Fabbri R., Kullolli O., Pallecchi M., Paradisi R., Danza G., Macciocca M., Lombardelli L., Seracchioli R. Description of the follicular fluid cytokine and hormone profiles in human physiological natural cycles. J. Clin. Endocrinol. Metabol. 2021;106:e721–e738. doi: 10.1210/clinem/dgaa880. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

