Inside-to-outside and back to the future of megakaryopoiesis
- PMID: 37416054
- PMCID: PMC10320384
- DOI: 10.1016/j.rpth.2023.100197
Inside-to-outside and back to the future of megakaryopoiesis
Abstract
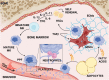
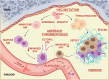
A State of the Art lecture titled "Megakaryocytes and different thrombopoietic environments" was presented at the ISTH Congress in 2022. Circulating platelets are specialized cells produced by megakaryocytes. Leading studies point to the bone marrow niche as the core of hematopoietic stem cell differentiation, revealing interesting and complex environmental factors for consideration. Megakaryocytes take cues from the physiochemical bone marrow microenvironment, which includes cell-cell interactions, contact with extracellular matrix components, and flow generated by blood circulation in the sinusoidal lumen. Germinal and acquired mutations in hematopoietic stem cells may manifest in altered megakaryocyte maturation, proliferation, and platelet production. Diseased megakaryopoiesis may also cause modifications of the entire hematopoietic niche, highlighting the central role of megakaryocytes in the control of physiologic bone marrow homeostasis. Tissue-engineering approaches have been developed to translate knowledge from in vivo (inside) to functional mimics of native tissue ex vivo (outside). Reproducing the thrombopoietic environment is instrumental to gain new insight into its activity and answering the growing demand for human platelets for fundamental studies and clinical applications. In this review, we discuss the major achievements on this topic, and finally, we summarize relevant new data presented during the 2022 ISTH Congress that pave the road to the future of megakaryopoiesis.
Keywords: bone marrow; megakaryocytes; personalized therapy; platelets; thrombocytopenia.
© 2023 The Author(s).
Figures


Similar articles
-
Immune attack on megakaryocytes in immune thrombocytopenia.Res Pract Thromb Haemost. 2024 Mar 14;8(1):102345. doi: 10.1016/j.rpth.2024.102345. eCollection 2024 Jan. Res Pract Thromb Haemost. 2024. PMID: 38525349 Free PMC article.
-
Miniaturized 3D bone marrow tissue model to assess response to Thrombopoietin-receptor agonists in patients.Elife. 2021 Jun 1;10:e58775. doi: 10.7554/eLife.58775. Elife. 2021. PMID: 34059198 Free PMC article.
-
Megakaryocytes in the lung: History and future perspectives.Res Pract Thromb Haemost. 2023 Jan 19;7(2):100053. doi: 10.1016/j.rpth.2023.100053. eCollection 2023 Feb. Res Pract Thromb Haemost. 2023. PMID: 37063766 Free PMC article.
-
Blood platelet production and morphology.Thromb Res. 2012 Mar;129(3):241-4. doi: 10.1016/j.thromres.2011.11.042. Epub 2012 Jan 5. Thromb Res. 2012. PMID: 22226434 Review.
-
Bone marrow niche in immune thrombocytopenia: a focus on megakaryopoiesis.Ann Hematol. 2016 Oct;95(11):1765-76. doi: 10.1007/s00277-016-2703-1. Epub 2016 May 28. Ann Hematol. 2016. PMID: 27236577 Review.
Cited by
-
Bioprinting Soft 3D Models of Hematopoiesis using Natural Silk Fibroin-Based Bioink Efficiently Supports Platelet Differentiation.Adv Sci (Weinh). 2024 May;11(18):e2308276. doi: 10.1002/advs.202308276. Epub 2024 Mar 21. Adv Sci (Weinh). 2024. PMID: 38514919 Free PMC article.
-
Immune and Inflammatory Properties of Megakaryocytes.Cells. 2025 Jul 10;14(14):1053. doi: 10.3390/cells14141053. Cells. 2025. PMID: 40710306 Free PMC article. Review.
-
A modern overview of the process of platelet formation (thrombocytopoiesis) and its dependence on several factors.Biochem Med (Zagreb). 2024 Oct 15;34(3):030503. doi: 10.11613/BM.2024.030503. Biochem Med (Zagreb). 2024. PMID: 39435166 Free PMC article. Review.
-
Revisiting Circulating Extracellular Matrix Fragments as Disease Markers in Myelofibrosis and Related Neoplasms.Cancers (Basel). 2023 Aug 29;15(17):4323. doi: 10.3390/cancers15174323. Cancers (Basel). 2023. PMID: 37686599 Free PMC article. Review.
References
-
- Nakamura-Ishizu A., Matsumura T., Stumpf P.S., Umemoto T., Takizawa H., Takihara Y., et al. Thrombopoietin metabolically primes hematopoietic stem cells to megakaryocyte-lineage differentiation. Cell Rep. 2018;25:1772–1785. - PubMed
LinkOut - more resources
Full Text Sources

